Neuroimmunological blood brain barrier opening in experimental cerebral malaria
- PMID: 23133375
- PMCID: PMC3486917
- DOI: 10.1371/journal.ppat.1002982
Neuroimmunological blood brain barrier opening in experimental cerebral malaria
Abstract
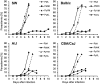
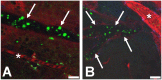
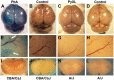
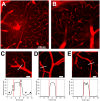
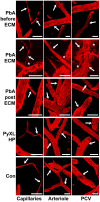
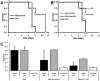
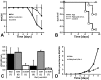
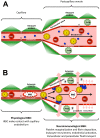
Plasmodium falciparum malaria is responsible for nearly one million annual deaths worldwide. Because of the difficulty in monitoring the pathogenesis of cerebral malaria in humans, we conducted a study in various mouse models to better understand disease progression in experimental cerebral malaria (ECM). We compared the effect on the integrity of the blood brain barrier (BBB) and the histopathology of the brain of P. berghei ANKA, a known ECM model, P. berghei NK65, generally thought not to induce ECM, P. yoelii 17XL, originally reported to induce human cerebral malaria-like histopathology, and P. yoelii YM. As expected, P. berghei ANKA infection caused neurological signs, cerebral hemorrhages, and BBB dysfunction in CBA/CaJ and Swiss Webster mice, while Balb/c and A/J mice were resistant. Surprisingly, PbNK induced ECM in CBA/CaJ mice, while all other mice were resistant. P. yoelii 17XL and P. yoelii YM caused lethal hyperparasitemia in all mouse strains; histopathological alterations, BBB dysfunction, or neurological signs were not observed. Intravital imaging revealed that infected erythrocytes containing mature parasites passed slowly through capillaries making intimate contact with the endothelium, but did not arrest. Except for relatively rare microhemorrhages, mice with ECM presented no obvious histopathological alterations that would explain the widespread disruption of the BBB. Intravital imaging did reveal, however, that postcapillary venules, but not capillaries or arterioles, from mice with ECM, but not hyperparasitemia, exhibit platelet marginalization, extravascular fibrin deposition, CD14 expression, and extensive vascular leakage. Blockage of LFA-1 mediated cellular interactions prevented leukocyte adhesion, vascular leakage, neurological signs, and death from ECM. The endothelial barrier-stabilizing mediators imatinib and FTY720 inhibited vascular leakage and neurological signs and prolonged survival to ECM. Thus, it appears that neurological signs and coma in ECM are due to regulated opening of paracellular-junctional and transcellular-vesicular fluid transport pathways at the neuroimmunological BBB.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Rogerson SJ, Grau GE, Hunt NH (2004) The microcirculation in severe malaria. Microcirculation 11: 559–576. - PubMed
-
- Newton CR, Taylor TE, Whitten RO (1998) Pathophysiology of fatal falciparum malaria in African children. Am J Trop Med Hyg 58: 673–683. - PubMed
-
- WHO (2000) Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Trans R Soc Trop Med Hyg 94 Suppl 1: S1–90. - PubMed
-
- Haldar K, Murphy SC, Milner DA, Taylor TE (2007) Malaria: mechanisms of erythrocytic infection and pathological correlates of severe disease. Annu Rev Pathol 2: 217–249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

