Glycoprotein N of human cytomegalovirus protects the virus from neutralizing antibodies
- PMID: 23133379
- PMCID: PMC3486915
- DOI: 10.1371/journal.ppat.1002999
Glycoprotein N of human cytomegalovirus protects the virus from neutralizing antibodies
Abstract
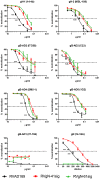
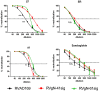
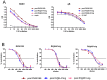
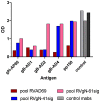
Herpes viruses persist in the infected host and are transmitted between hosts in the presence of a fully functional humoral immune response, suggesting that they can evade neutralization by antiviral antibodies. Human cytomegalovirus (HCMV) encodes a number of polymorphic highly glycosylated virion glycoproteins (g), including the essential envelope glycoprotein, gN. We have tested the hypothesis that glycosylation of gN contributes to resistance of the virus to neutralizing antibodies. Recombinant viruses carrying deletions in serine/threonine rich sequences within the glycosylated surface domain of gN were constructed in the genetic background of HCMV strain AD169. The deletions had no influence on the formation of the gM/gN complex and in vitro replication of the respective viruses compared to the parent virus. The gN-truncated viruses were significantly more susceptible to neutralization by a gN-specific monoclonal antibody and in addition by a number of gB- and gH-specific monoclonal antibodies. Sera from individuals previously infected with HCMV also more efficiently neutralized gN-truncated viruses. Immunization of mice with viruses that expressed the truncated forms of gN resulted in significantly higher serum neutralizing antibody titers against the homologous strain that was accompanied by increased antibody titers against known neutralizing epitopes on gB and gH. Importantly, neutralization activity of sera from animals immunized with gN-truncated virus did not exhibit enhanced neutralizing activity against the parental wild type virus carrying the fully glycosylated wild type gN. Our results indicate that the extensive glycosylation of gN could represent a potentially important mechanism by which HCMV neutralization by a number of different antibody reactivities can be inhibited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Jackson SE, Mason GM, Wills MR (2011) Human cytomegalovirus immunity and immune evasion. Virus Res 157: 151–160. - PubMed
-
- Reddehase MJ (2002) Antigens and immunoevasins: opponents in cytomegalovirus immune surveillance. Nat Rev Immunol 2: 831–844. - PubMed
-
- Smith LM, Shellam GR, Redwood AJ (2006) Genes of murine cytomegalovirus exist as a number of distinct genotypes. Virology 352: 450–465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

