Disruption of CHTF18 causes defective meiotic recombination in male mice
- PMID: 23133398
- PMCID: PMC3486840
- DOI: 10.1371/journal.pgen.1002996
Disruption of CHTF18 causes defective meiotic recombination in male mice
Abstract
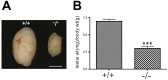
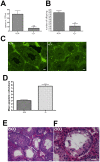
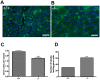
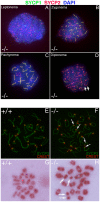
CHTF18 (chromosome transmission fidelity factor 18) is an evolutionarily conserved subunit of the Replication Factor C-like complex, CTF18-RLC. CHTF18 is necessary for the faithful passage of chromosomes from one daughter cell to the next during mitosis in yeast, and it is crucial for germline development in the fruitfly. Previously, we showed that mouse Chtf18 is expressed throughout the germline, suggesting a role for CHTF18 in mammalian gametogenesis. To determine the role of CHTF18 in mammalian germ cell development, we derived mice carrying null and conditional mutations in the Chtf18 gene. Chtf18-null males exhibit 5-fold decreased sperm concentrations compared to wild-type controls, resulting in subfertility. Loss of Chtf18 results in impaired spermatogenesis; spermatogenic cells display abnormal morphology, and the stereotypical arrangement of cells within seminiferous tubules is perturbed. Meiotic recombination is defective and homologous chromosomes separate prematurely during prophase I. Repair of DNA double-strand breaks is delayed and incomplete; both RAD51 and γH2AX persist in prophase I. In addition, MLH1 foci are decreased in pachynema. These findings demonstrate essential roles for CHTF18 in mammalian spermatogenesis and meiosis, and suggest that CHTF18 may function during the double-strand break repair pathway to promote the formation of crossovers.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
CHTF18 ensures the quantity and quality of the ovarian reserve†.Biol Reprod. 2020 Jun 23;103(1):24-35. doi: 10.1093/biolre/ioaa036. Biol Reprod. 2020. PMID: 32219340 Free PMC article.
-
TopBP1 deficiency impairs the localization of proteins involved in early recombination and results in meiotic chromosome defects during spermatogenesis.Biochem Biophys Res Commun. 2019 Jan 15;508(3):722-728. doi: 10.1016/j.bbrc.2018.12.001. Epub 2018 Dec 7. Biochem Biophys Res Commun. 2019. PMID: 30528234
-
Immunofluorescent characterization of meiotic recombination in human males with variable spermatogenesis.Andrology. 2013 Mar;1(2):262-73. doi: 10.1111/j.2047-2927.2012.00039.x. Epub 2012 Nov 29. Andrology. 2013. PMID: 23413139
-
Genetics of meiosis and recombination in mice.Int Rev Cell Mol Biol. 2012;298:179-227. doi: 10.1016/B978-0-12-394309-5.00005-5. Int Rev Cell Mol Biol. 2012. PMID: 22878107 Review.
-
Mechanisms of meiosis initiation and meiotic prophase progression during spermatogenesis.Mol Aspects Med. 2024 Jun;97:101282. doi: 10.1016/j.mam.2024.101282. Epub 2024 May 25. Mol Aspects Med. 2024. PMID: 38797021 Review.
Cited by
-
Eukaryotic clamp loaders and unloaders in the maintenance of genome stability.Exp Mol Med. 2020 Dec;52(12):1948-1958. doi: 10.1038/s12276-020-00533-3. Epub 2020 Dec 18. Exp Mol Med. 2020. PMID: 33339954 Free PMC article. Review.
-
Application of neural network-based image analysis to detect sister chromatid cohesion defects.Sci Rep. 2023 Feb 6;13(1):2133. doi: 10.1038/s41598-023-28742-6. Sci Rep. 2023. PMID: 36747022 Free PMC article.
-
Differential isoform expression and alternative splicing in sex determination in mice.BMC Genomics. 2019 Mar 12;20(1):202. doi: 10.1186/s12864-019-5572-x. BMC Genomics. 2019. PMID: 30871468 Free PMC article.
-
Disruption of the moonlighting function of CTF18 in a patient with T-lymphopenia.Front Immunol. 2025 Feb 14;16:1539848. doi: 10.3389/fimmu.2025.1539848. eCollection 2025. Front Immunol. 2025. PMID: 40028343 Free PMC article.
-
CHTF18 ensures the quantity and quality of the ovarian reserve†.Biol Reprod. 2020 Jun 23;103(1):24-35. doi: 10.1093/biolre/ioaa036. Biol Reprod. 2020. PMID: 32219340 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA082710/CA/NCI NIH HHS/United States
- R01 GM076327/GM/NIGMS NIH HHS/United States
- R01 GM106262/GM/NIGMS NIH HHS/United States
- P01-CA082710/CA/NCI NIH HHS/United States
- GM076327/GM/NIGMS NIH HHS/United States
- K12 HD001256/HD/NICHD NIH HHS/United States
- HD 34449/HD/NICHD NIH HHS/United States
- HD 01256/HD/NICHD NIH HHS/United States
- P30 DK050306/DK/NIDDK NIH HHS/United States
- U54 HD034449/HD/NICHD NIH HHS/United States
- R01 HD033834/HD/NICHD NIH HHS/United States
- P30 DK 50306/DK/NIDDK NIH HHS/United States
- HD 33834/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

