Trps1 and its target gene Sox9 regulate epithelial proliferation in the developing hair follicle and are associated with hypertrichosis
- PMID: 23133399
- PMCID: PMC3486859
- DOI: 10.1371/journal.pgen.1003002
Trps1 and its target gene Sox9 regulate epithelial proliferation in the developing hair follicle and are associated with hypertrichosis
Abstract
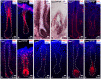
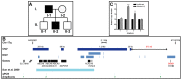
Hereditary hypertrichoses are a group of hair overgrowth syndromes that are extremely rare in humans. We have previously demonstrated that a position effect on TRPS1 is associated with hypertrichosis in humans and mice. To gain insight into the functional role of Trps1, we analyzed the late morphogenesis vibrissae phenotype of Trps1(Δgt) mutant mice, which is characterized by follicle degeneration after peg downgrowth has been initiated. We found that Trps1 directly represses expression of the hair follicle stem cell regulator Sox9 to control proliferation of the follicle epithelium. Furthermore, we identified a copy number variation upstream of SOX9 in a family with hypertrichosis that significantly decreases expression of the gene in the hair follicle, providing new insights into the long-range regulation of SOX9. Our findings uncover a novel transcriptional hierarchy that regulates epithelial proliferation in the developing hair follicle and contributes to the pathology of hypertrichosis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Garcia-Cruz D, Figuera LE, Cantu JM (2002) Inherited hypertrichoses. Clin Genet 61: 321–329. - PubMed
-
- Beighton P (1970) Congenital hypertrichosis lanuginosa. Arch Dermatol 101: 669–672. - PubMed
-
- Baumeister FA, Egger J, Schildhauer MT, Stengel-Rutkowski S (1993) Ambras syndrome: delineation of a unique hypertrichosis universalis congenita and association with a balanced pericentric inversion (8) (p11.2; q22). Clin Genet 44: 121–128. - PubMed
-
- Macías-Flores MA, García-Cruz D, Rivera H, Escobar-Luján M, Melendrez-Vega A, et al. (1984) A new form of hypertrichosis inherited as an X-linked dominant trait. Hum Genet 66: 66–70. - PubMed
-
- Canun S, Guevara-Sangines EG, Elvira-Morales A, Sierra-Romero Mdel C, Rodriguez-Asbun H (2003) Hypertrichosis terminalis, gingival hyperplasia, and a characteristic face: a new distinct entity. Am J Med Genet A 116A: 278–283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

