A transcriptome-wide screen for mRNAs enriched in fetal Leydig cells: CRHR1 agonism stimulates rat and mouse fetal testis steroidogenesis
- PMID: 23133512
- PMCID: PMC3484991
- DOI: 10.1371/journal.pone.0047359
A transcriptome-wide screen for mRNAs enriched in fetal Leydig cells: CRHR1 agonism stimulates rat and mouse fetal testis steroidogenesis
Abstract
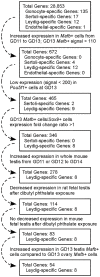
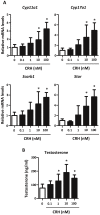
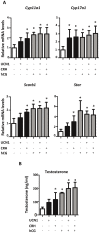
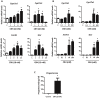
Fetal testis steroidogenesis plays an important role in the reproductive development of the male fetus. While regulators of certain aspects of steroidogenesis are known, the initial driver of steroidogenesis in the human and rodent fetal testis is unclear. Through comparative analysis of rodent fetal testis microarray datasets, 54 candidate fetal Leydig cell-specific genes were identified. Fetal mouse testis interstitial expression of a subset of these genes with unknown expression (Crhr1, Gramd1b, Itih5, Vgll3, and Vsnl1) was verified by whole-mount in situ hybridization. Among the candidate fetal Leydig cell-specific factors, three receptors (CRHR1, PRLR, and PROKR2) were tested for a steroidogenic function using ex vivo fetal testes treated with receptor agonists (CRH, PRL, and PROK2). While PRL and PROK2 had no effect, CRH, at low (approximately 1 to 10) nM concentration, increased expression of the steroidogenic genes Cyp11a1, Cyp17a1, Scarb1, and Star in GD15 mouse and GD17 rat testes, and in conjunction, testosterone production was increased. Exposure of GD15 fetal mouse testis to a specific CRHR1 antagonist blunted the CRH-induced steroidogenic gene expression and testosterone responses. Similar to ex vivo rodent fetal testes, ≥ 10 nM CRH exposure of MA-10 Leydig cells increased steroidogenic pathway mRNA and progesterone levels, showing CRH can enhance steroidogenesis by directly targeting Leydig cells. Crh mRNA expression was observed in rodent fetal hypothalamus, and CRH peptide was detected in rodent amniotic fluid. Together, these data provide a resource for discovering factors controlling fetal Leydig cell biology and suggest that CRHR1 activation by CRH stimulates rat and mouse fetal Leydig cell steroidogenesis in vivo.
Conflict of interest statement
Figures



Similar articles
-
Corticotropin-releasing hormone stimulates steroidogenesis in mouse Leydig cells.Biol Reprod. 1995 Sep;53(3):620-6. doi: 10.1095/biolreprod53.3.620. Biol Reprod. 1995. PMID: 7578686
-
Single-cell resolution uncovers neighboring cell subtypes that share steroidogenic capacity during fetal testis development.Proc Natl Acad Sci U S A. 2025 Jun 10;122(23):e2501392122. doi: 10.1073/pnas.2501392122. Epub 2025 Jun 3. Proc Natl Acad Sci U S A. 2025. PMID: 40460128
-
Expression patterns of C1ql4 and its cell-adhesion GPCR Bai3 in the murine testis and functional roles in steroidogenesis.FASEB J. 2019 Apr;33(4):4893-4906. doi: 10.1096/fj.201801620RR. Epub 2019 Jan 4. FASEB J. 2019. PMID: 30608882
-
Fetal Leydig cells: What we know and what we don't.Mol Reprod Dev. 2024 Mar;91(3):e23739. doi: 10.1002/mrd.23739. Mol Reprod Dev. 2024. PMID: 38480999 Free PMC article. Review.
-
Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds.Endocr Rev. 2009 Dec;30(7):883-925. doi: 10.1210/er.2009-0016. Epub 2009 Nov 3. Endocr Rev. 2009. PMID: 19887492 Review.
Cited by
-
Heterogeneity of ovarian theca and interstitial gland cells in mice.PLoS One. 2015 Jun 3;10(6):e0128352. doi: 10.1371/journal.pone.0128352. eCollection 2015. PLoS One. 2015. PMID: 26039146 Free PMC article.
-
From the Cover: Teratogenic Effects of in Utero Exposure to Di-(2-Ethylhexyl)-Phthalate (DEHP) in B6:129S4 Mice.Toxicol Sci. 2017 May 1;157(1):8-19. doi: 10.1093/toxsci/kfx019. Toxicol Sci. 2017. PMID: 28123099 Free PMC article.
-
Aster-B coordinates with Arf1 to regulate mitochondrial cholesterol transport.Mol Metab. 2020 Dec;42:101055. doi: 10.1016/j.molmet.2020.101055. Epub 2020 Jul 29. Mol Metab. 2020. PMID: 32738348 Free PMC article.
-
Ltc1 is an ER-localized sterol transporter and a component of ER-mitochondria and ER-vacuole contacts.J Cell Biol. 2015 May 25;209(4):539-48. doi: 10.1083/jcb.201502033. Epub 2015 May 18. J Cell Biol. 2015. PMID: 25987606 Free PMC article.
-
Vgll3 and the Hippo pathway are regulated in Sertoli cells upon entry and during puberty in Atlantic salmon testis.Sci Rep. 2018 Jan 30;8(1):1912. doi: 10.1038/s41598-018-20308-1. Sci Rep. 2018. PMID: 29382956 Free PMC article.
References
-
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R (1991) Male development of chromosomally female mice transgenic for Sry. Nature 351: 117–121. - PubMed
-
- Koopman P, Munsterberg A, Capel B, Vivian N, Lovell-Badge R (1990) Expression of a candidate sex-determining gene during mouse testis differentiation. Nature 348: 450–452. - PubMed
-
- Kent J, Wheatley SC, Andrews JE, Sinclair AH, Koopman P (1996) A male-specific role for SOX9 in vertebrate sex determination. Development 122: 2813–2822. - PubMed
-
- Morais da Silva S, Hacker A, Harley V, Goodfellow P, Swain A, et al. (1996) Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat Genet 14: 62–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

