Gatifloxacin induces S and G2-phase cell cycle arrest in pancreatic cancer cells via p21/p27/p53
- PMID: 23133524
- PMCID: PMC3485023
- DOI: 10.1371/journal.pone.0047796
Gatifloxacin induces S and G2-phase cell cycle arrest in pancreatic cancer cells via p21/p27/p53
Abstract
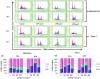
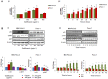
Pancreatic cancer, despite being the most dreadful among gastrointestinal cancers, is poorly diagnosed, and further, the situation has been aggravated owing to acquired drug resistance against the single known drug therapy. While previous studies have highlighted the growth inhibitory effects of older generation fluoroquinolones, the current study aims to evaluate the growth inhibitory effects of newer generation fluoroquinolone, Gatifloxacin, on pancreatic cancer cell lines MIA PaCa-2 and Panc-1 as well as to elucidate the underlying molecular mechanisms. Herein, we report that Gatifloxacin suppresses the proliferation of MIA PaCa-2 and Panc-1 cells by causing S and G(2)-phase cell cycle arrest without induction of apoptosis. Blockade in S-phase of the cell cycle was associated with increased TGF-β1 expression and translocation of Smad3-4 complex to the nucleus with subsequent activation of p21 in MIA PaCa-2 cells, whereas TGF-β signalling attenuated Panc-1 cells showed S-phase arrest by direct activation of p27. However, Gatifloxacin mediated G(2)-phase cell cycle arrest was found to be p53 dependent in both the cell lines. Our study is of interest because fluoroquinolones have the ability to penetrate pancreatic tissue which can be very effective in combating pancreatic cancers that are usually associated with loss or downregulation of CDK inhibitors p21/p27 as well as mutational inactivation of p53. Additionally, Gatifloxacin was also found to synergize the effect of Gemcitabine, the only known drug against pancreatic cancer, as well as the broad spectrum anticancer drug cisplatin. Taken together our results suggest that Gatifloxacin possesses anticancer activities against pancreatic cancer and is a promising candidate to be repositioned from broad spectrum antibiotics to anticancer agent.
Conflict of interest statement
Figures






Similar articles
-
Moxifloxacin and ciprofloxacin induces S-phase arrest and augments apoptotic effects of cisplatin in human pancreatic cancer cells via ERK activation.BMC Cancer. 2015 Aug 11;15:581. doi: 10.1186/s12885-015-1560-y. BMC Cancer. 2015. PMID: 26260159 Free PMC article.
-
Leukotriene B4 receptor antagonist LY293111 induces S-phase cell cycle arrest and apoptosis in human pancreatic cancer cells.Anticancer Drugs. 2007 Jun;18(5):535-41. doi: 10.1097/01.cad.0000231477.22901.8a. Anticancer Drugs. 2007. PMID: 17414622
-
Caffeic acid phenethyl ester induced cell cycle arrest and growth inhibition in androgen-independent prostate cancer cells via regulation of Skp2, p53, p21Cip1 and p27Kip1.Oncotarget. 2015 Mar 30;6(9):6684-707. doi: 10.18632/oncotarget.3246. Oncotarget. 2015. PMID: 25788262 Free PMC article.
-
Cell cycle regulation by the intrinsically disordered proteins p21 and p27.Biochem Soc Trans. 2012 Oct;40(5):981-8. doi: 10.1042/BST20120092. Biochem Soc Trans. 2012. PMID: 22988851 Free PMC article. Review.
-
The potential roles of p53 signaling reactivation in pancreatic cancer therapy.Biochim Biophys Acta Rev Cancer. 2022 Jan;1877(1):188662. doi: 10.1016/j.bbcan.2021.188662. Epub 2021 Nov 30. Biochim Biophys Acta Rev Cancer. 2022. PMID: 34861354 Review.
Cited by
-
Recent Development of Fluoroquinolone Derivatives as Anticancer Agents.Molecules. 2024 Jul 27;29(15):3538. doi: 10.3390/molecules29153538. Molecules. 2024. PMID: 39124943 Free PMC article. Review.
-
Moxifloxacin and ciprofloxacin induces S-phase arrest and augments apoptotic effects of cisplatin in human pancreatic cancer cells via ERK activation.BMC Cancer. 2015 Aug 11;15:581. doi: 10.1186/s12885-015-1560-y. BMC Cancer. 2015. PMID: 26260159 Free PMC article.
-
In Vitro Growth Inhibition, Caspase-Dependent Apoptosis, and S and G2/M Phase Arrest in Breast Cancer Cells Induced by Fluorine-Incorporated Gold I Compound, Ph3PAu[SC(OMe)=NC6H4F-3].Int J Breast Cancer. 2022 Jul 21;2022:7168210. doi: 10.1155/2022/7168210. eCollection 2022. Int J Breast Cancer. 2022. PMID: 35910309 Free PMC article.
-
Berbamine Suppresses the Progression of Bladder Cancer by Modulating the ROS/NF-κB Axis.Oxid Med Cell Longev. 2021 Jan 13;2021:8851763. doi: 10.1155/2021/8851763. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33520087 Free PMC article.
-
NFκB activation demarcates a subset of hepatocellular carcinoma patients for targeted therapy.Cell Oncol (Dordr). 2016 Dec;39(6):523-536. doi: 10.1007/s13402-016-0294-4. Epub 2016 Aug 25. Cell Oncol (Dordr). 2016. PMID: 27562587
References
-
- Apte MV, Park S, Phillips PA, Santucci N, Goldstein D, et al. (2004) Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas 29: 179–187. - PubMed
-
- Riall TS, Cameron JL, Lillemoe KD, Winter JM, Campbell KA, et al. (2006) Resected periampullary adenocarcinoma: 5-year survivors and their 6- to 10-year follow-up. Surgery 140: 764–772. - PubMed
-
- Bao PQ, Ramanathan RK, Krasinkas A, Bahary N, Lembersky BC, et al... (2011) III, Erratum to: Phase II Study of Gemcitabine and Erlotinib as Adjuvant Therapy for Patients with Resected Pancreatic Cancer Ann Surg Oncol. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

