Mechanical disruption of tumors by iron particles and magnetic field application results in increased anti-tumor immune responses
- PMID: 23133545
- PMCID: PMC3485005
- DOI: 10.1371/journal.pone.0048049
Mechanical disruption of tumors by iron particles and magnetic field application results in increased anti-tumor immune responses
Abstract
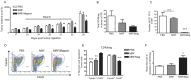
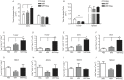
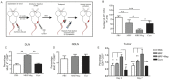
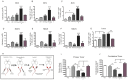
The primary tumor represents a potential source of antigens for priming immune responses for disseminated disease. Current means of debulking tumors involves the use of cytoreductive conditioning that impairs immune cells or removal by surgery. We hypothesized that activation of the immune system could occur through the localized release of tumor antigens and induction of tumor death due to physical disruption of tumor architecture and destruction of the primary tumor in situ. This was accomplished by intratumor injection of magneto-rheological fluid (MRF) consisting of iron microparticles, in Balb/c mice bearing orthotopic 4T1 breast cancer, followed by local application of a magnetic field resulting in immediate coalescence of the particles, tumor cell death, slower growth of primary tumors as well as decreased tumor progression in distant sites and metastatic spread. This treatment was associated with increased activation of DCs in the draining lymph nodes and recruitment of both DCs and CD8(+)T cells to the tumor. The particles remained within the tumor and no toxicities were observed. The immune induction observed was significantly greater compared to cryoablation. Further anti-tumor effects were observed when MRF/magnet therapy was combined with systemic low dose immunotherapy. Thus, mechanical disruption of the primary tumor with MRF/magnetic field application represents a novel means to induce systemic immune activation in cancer.
Conflict of interest statement
Figures


References
-
- Curley SA (2003) Radiofrequency ablation of malignant liver tumors. Ann Surg Oncol 10: 338–347. - PubMed
-
- Raj GV, Reddan DJ, Hoey MF, Polascik TJ (2003) Management of small renal tumors with radiofrequency ablation. Urology 61: 23–29. - PubMed
-
- Zhao Z, Wu F (2010) Minimally-invasive thermal ablation of early-stage breast cancer: a systemic review. Eur J Surg Oncol 36: 1149–1155. - PubMed
-
- Garcea G, Lloyd TD, Aylott C, Maddern G, Berry DP (2003) The emergent role of focal liver ablation techniques in the treatment of primary and secondary liver tumours. Eur J Cancer 39: 2150–2164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

