Rubber elongation factor (REF), a major allergen component in Hevea brasiliensis latex has amyloid properties
- PMID: 23133547
- PMCID: PMC3485013
- DOI: 10.1371/journal.pone.0048065
Rubber elongation factor (REF), a major allergen component in Hevea brasiliensis latex has amyloid properties
Abstract
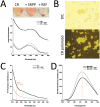
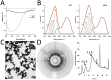
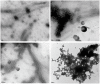
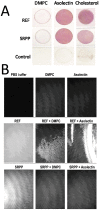
REF (Hevb1) and SRPP (Hevb3) are two major components of Hevea brasiliensis latex, well known for their allergenic properties. They are obviously taking part in the biosynthesis of natural rubber, but their exact function is still unclear. They could be involved in defense/stress mechanisms after tapping or directly acting on the isoprenoid biosynthetic pathway. The structure of these two proteins is still not described. In this work, it was discovered that REF has amyloid properties, contrary to SRPP. We investigated their structure by CD, TEM, ATR-FTIR and WAXS and neatly showed the presence of β-sheet organized aggregates for REF, whereas SRPP mainly fold as a helical protein. Both proteins are highly hydrophobic but differ in their interaction with lipid monolayers used to mimic the monomembrane surrounding the rubber particles. Ellipsometry experiments showed that REF seems to penetrate deeply into the monolayer and SRPP only binds to the lipid surface. These results could therefore clarify the role of these two paralogous proteins in latex production, either in the coagulation of natural rubber or in stress-related responses. To our knowledge, this is the first report of an amyloid formed from a plant protein. This suggests also the presence of functional amyloid in the plant kingdom.
Conflict of interest statement
Figures


References
-
- van Beilen JB, Poirier Y (2007) Establishment of new crops for the production of natural rubber. Trends Biotechnol 25: 522–529. - PubMed
-
- d’ Auzac J, Jacob JL, Chrestin H (1989) The composition oh latex from Hevea brasiliensis as laticiferous cytoplasm. In: d’ Auzac J, Jacob JL, editors. Physiology of the rubber tree latex. Boca Raton, Florida: CRC Press.
-
- Akasawa A, Hsieh LS, Lin Y (1995) Serum reactivities to latex proteins (Hevea brasiliensis). J Allergy Clin Immunol 95: 1196–1205. - PubMed
-
- Czuppon AB, Chen Z, Rennert S, Engelke T, Meyer HE, et al. (1993) The rubber elongation factor of rubber trees (Hevea brasiliensis) is the major allergen in latex. J Allergy Clin Immunol 92: 690–697. - PubMed
-
- Wagner B, Krebitz M, Buck D, Niggemann B, Yeang HY, et al. (1999) Cloning, expression, and characterization of recombinant Hev b 3, a Hevea brasiliensis protein associated with latex allergy in patients with spina bifida. J Allergy Clin Immunol 104: 1084–1092. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

