The homeobox BcHOX8 gene in Botrytis cinerea regulates vegetative growth and morphology
- PMID: 23133556
- PMCID: PMC3485016
- DOI: 10.1371/journal.pone.0048134
The homeobox BcHOX8 gene in Botrytis cinerea regulates vegetative growth and morphology
Abstract
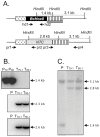
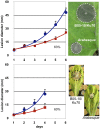
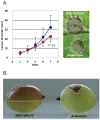
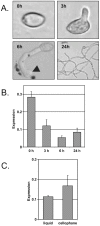
Filamentous growth and the capacity at producing conidia are two critical aspects of most fungal life cycles, including that of many plant or animal pathogens. Here, we report on the identification of a homeobox transcription factor encoding gene that plays a role in these two particular aspects of the development of the phytopathogenic fungus Botrytis cinerea. Deletion of the BcHOX8 gene in both the B. cinerea B05-10 and T4 strains causes similar phenotypes, among which a curved, arabesque-like, hyphal growth on hydrophobic surfaces; the mutants were hence named Arabesque. Expression of the BcHOX8 gene is higher in conidia and infection cushions than in developing appressorium or mycelium. In the Arabesque mutants, colony growth rate is reduced and abnormal infection cushions are produced. Asexual reproduction is also affected with abnormal conidiophore being formed, strongly reduced conidia production and dramatic changes in conidial morphology. Finally, the mutation affects the fungus ability to efficiently colonize different host plants. Analysis of the B. cinerea genome shows that BcHOX8 is one member of a nine putative homeobox genes family. Available gene expression data suggest that these genes are functional and sequence comparisons indicate that two of them would be specific to B. cinerea and its close relative Sclerotinia sclerotiorum.
Conflict of interest statement
Figures


Similar articles
-
A novel Botrytis cinerea-specific gene BcHBF1 enhances virulence of the grey mould fungus via promoting host penetration and invasive hyphal development.Mol Plant Pathol. 2019 May;20(5):731-747. doi: 10.1111/mpp.12788. Mol Plant Pathol. 2019. PMID: 31008573 Free PMC article.
-
Transcriptome profiling of Botrytis cinerea conidial germination reveals upregulation of infection-related genes during the prepenetration stage.Eukaryot Cell. 2013 Apr;12(4):614-26. doi: 10.1128/EC.00295-12. Epub 2013 Feb 15. Eukaryot Cell. 2013. PMID: 23417562 Free PMC article.
-
The Autophagy Gene BcATG8 Regulates the Vegetative Differentiation and Pathogenicity of Botrytis cinerea.Appl Environ Microbiol. 2018 May 17;84(11):e02455-17. doi: 10.1128/AEM.02455-17. Print 2018 Jun 1. Appl Environ Microbiol. 2018. PMID: 29572212 Free PMC article.
-
Recent Advances in the Study of the Plant Pathogenic Fungus Botrytis cinerea and its Interaction with the Environment.Curr Protein Pept Sci. 2017;18(10):976-989. doi: 10.2174/1389203717666160809160915. Curr Protein Pept Sci. 2017. PMID: 27526927 Review.
-
Emerging Trends in Molecular Interactions between Plants and the Broad Host Range Fungal Pathogens Botrytis cinerea and Sclerotinia sclerotiorum.Front Plant Sci. 2016 Mar 31;7:422. doi: 10.3389/fpls.2016.00422. eCollection 2016. Front Plant Sci. 2016. PMID: 27066056 Free PMC article. Review.
Cited by
-
A secreted metal-binding protein protects necrotrophic phytopathogens from reactive oxygen species.Nat Commun. 2019 Oct 24;10(1):4853. doi: 10.1038/s41467-019-12826-x. Nat Commun. 2019. PMID: 31649262 Free PMC article.
-
FgHtf1 Regulates Global Gene Expression towards Aerial Mycelium and Conidiophore Formation in the Cereal Fungal Pathogen Fusarium graminearum.Appl Environ Microbiol. 2020 Apr 17;86(9):e03024-19. doi: 10.1128/AEM.03024-19. Print 2020 Apr 17. Appl Environ Microbiol. 2020. PMID: 32086302 Free PMC article.
-
The GRF10 homeobox gene regulates filamentous growth in the human fungal pathogen Candida albicans.FEMS Yeast Res. 2015 Dec;15(8):fov093. doi: 10.1093/femsyr/fov093. Epub 2015 Oct 15. FEMS Yeast Res. 2015. PMID: 26472755 Free PMC article.
-
Clathrin Is Important for Virulence Factors Delivery in the Necrotrophic Fungus Botrytis cinerea.Front Plant Sci. 2021 Jun 16;12:668937. doi: 10.3389/fpls.2021.668937. eCollection 2021. Front Plant Sci. 2021. PMID: 34220891 Free PMC article.
-
Regulatory functions of homeobox domain transcription factors in fungi.Appl Environ Microbiol. 2024 Mar 20;90(3):e0220823. doi: 10.1128/aem.02208-23. Epub 2024 Feb 29. Appl Environ Microbiol. 2024. PMID: 38421174 Free PMC article. Review.
References
-
- Williamson B, Tudzynski B, Tudzynski P, van Kan J (2007) Botrytis cinerea: the cause of grey mould disease. Mol Plant Pathol 8: 561–580. - PubMed
-
- Choquer M, Fournier E, Kunz C, Levis C, Pradier J-M, et al. (2007) Botrytis cinerea virulence factors: new insights into a necrotrophic and polyphageous pathogen. FEMS Microbiology Letters 277: 1–10. - PubMed
-
- van Kan J (2006) Licensed to kill: the lifestyle of a necrotrophic plant pathogen. Trends Plant Sci 11: 247–253. - PubMed
-
- Rolland S, Jobic C, Fèvre M, Bruel C (2003) Agrobacterium-mediated transformation of Botrytis cinerea, simple purification of monokaryotic transformants and rapid conidia-based identification of the transfer-DNA host genomic DNA flanking sequences. Curr Genet 44: 164–171. - PubMed
-
- Patel R, van Kan J, Bailey A, Foster G (2008) RNA-mediated gene silencing of superoxide dismutase (bcsod1) in Botrytis cinerea . Phytopathology 98: 1334–1339. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

