Pregnancy induces transcriptional activation of the peripheral innate immune system and increases oxidative DNA damage among healthy third trimester pregnant women
- PMID: 23133592
- PMCID: PMC3487782
- DOI: 10.1371/journal.pone.0046736
Pregnancy induces transcriptional activation of the peripheral innate immune system and increases oxidative DNA damage among healthy third trimester pregnant women
Abstract
Background: Pregnancy induces physiological adaptations that may involve, or contribute to, alterations in the genomic landscape. Pregnancy also increases the nutritional demand for choline, an essential nutrient that can modulate epigenomic and transcriptomic readouts secondary to its role as a methyl donor. Nevertheless, the interplay between human pregnancy, choline and the human genome is largely unexplored.
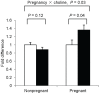
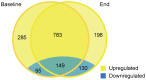
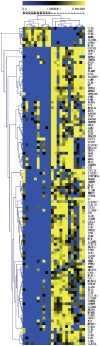
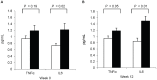
Methodology/principal findings: As part of a controlled feeding study, we assessed the influence of pregnancy and choline intake on maternal genomic markers. Healthy third trimester pregnant (n = 26, wk 26-29 gestation) and nonpregnant (n = 21) women were randomized to choline intakes of 480 mg/day, approximating the Adequate Intake level, or 930 mg/day for 12-weeks. Blood leukocytes were acquired at study week 0 and study week 12 for microarray, DNA damage and global DNA/histone methylation measurements. A main effect of pregnancy that was independent of choline intake was detected on several of the maternal leukocyte genomic markers. Compared to nonpregnant women, third trimester pregnant women exhibited higher (P<0.05) transcript abundance of defense response genes associated with the innate immune system including pattern recognition molecules, neutrophil granule proteins and oxidases, complement proteins, cytokines and chemokines. Pregnant women also exhibited higher (P<0.001) levels of DNA damage in blood leukocytes, a genomic marker of oxidative stress. No effect of choline intake was detected on the maternal leukocyte genomic markers with the exception of histone 3 lysine 4 di-methylation which was lower among pregnant women in the 930 versus 480 mg/d choline intake group.
Conclusions: Pregnancy induces transcriptional activation of the peripheral innate immune system and increases oxidative DNA damage among healthy third trimester pregnant women.
Conflict of interest statement
Figures
References
-
- Wegmann TG, Lin H, Guilbert L, Mosmann TR (1993) Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today 14: 353–356. - PubMed
-
- Witkin SS, Linhares IM, Bongiovanni AM, Herway C, Skupski D (2011) Unique alterations in infection-induced immune activation during pregnancy. BJOG 118: 145–153. - PubMed
-
- Belo L, Santos-Silva A, Rocha S, Caslake M, Cooney J, et al. (2005) Fluctuations in C-reactive protein concentration and neutrophil activation during normal human pregnancy. Eur J Obstet Gynecol Reprod Biol 123: 46–51. - PubMed
-
- Crouch SP, Crocker IP, Fletcher J (1995) The effect of pregnancy on polymorphonuclear leukocyte function. J Immunol 155: 5436–5443. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

