Protection of rabbits and immunodeficient mice against lethal poxvirus infections by human monoclonal antibodies
- PMID: 23133652
- PMCID: PMC3487784
- DOI: 10.1371/journal.pone.0048706
Protection of rabbits and immunodeficient mice against lethal poxvirus infections by human monoclonal antibodies
Abstract
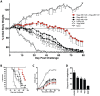
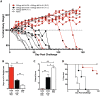
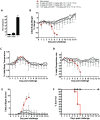
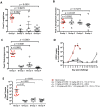
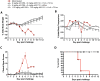
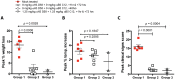
Smallpox (variola virus) is a bioweapon concern. Monkeypox is a growing zoonotic poxvirus threat. These problems have resulted in extensive efforts to develop potential therapeutics that can prevent or treat potentially lethal poxvirus infections in humans. Monoclonal antibodies (mAbs) against smallpox are a conservative approach to this problem, as the licensed human smallpox vaccine (vaccinia virus, VACV) primarily works on the basis of protective antibody responses against smallpox. Fully human mAbs (hmAbs) against vaccinia H3 (H3L) and B5 (B5R), targeting both the mature virion (MV) and extracellular enveloped virion (EV) forms, have been developed as potential therapeutics for use in humans. Post-exposure prophylaxis was assessed in both murine and rabbit animal models. Therapeutic efficacy of the mAbs was assessed in three good laboratory practices (GLP) studies examining severe combined immunodeficiency mice (SCID) given a lethal VACV infection. Pre-exposure combination hmAb therapy provided significantly better protection against disease and death than either single hmAb or vaccinia immune globulin (VIG). Post-exposure combination mAb therapy provided significant protection against disease and death, and appeared to fully cure the VACV infection in ≥50% of SCID mice. Therapeutic efficacy was then assessed in two rabbit studies examining post-exposure hmAb prophylaxis against rabbitpox (RPXV). In the first study, rabbits were infected with RPVX and then provided hmAbs at 48 hrs post-infection, or 1 hr and 72 hrs post-infection. Rabbits in both groups receiving hmAbs were 100% protected from death. In the second rabbitpox study, 100% of animal treated with combination hmAb therapy and 100% of animals treated with anti-B5 hmAb were protected. These findings suggest that combination hmAb treatment may be effective at controlling smallpox disease in immunocompetent or immunodeficient humans.
Conflict of interest statement
Figures


Similar articles
-
Combination therapy of vaccinia virus infection with human anti-H3 and anti-B5 monoclonal antibodies in a small animal model.Antivir Ther. 2010;15(4):661-75. doi: 10.3851/IMP1573. Antivir Ther. 2010. PMID: 20587859 Free PMC article.
-
Polyclonal antibody cocktails generated using DNA vaccine technology protect in murine models of orthopoxvirus disease.Virol J. 2011 Sep 20;8:441. doi: 10.1186/1743-422X-8-441. Virol J. 2011. PMID: 21933385 Free PMC article.
-
A Nucleic Acid-Based Orthopoxvirus Vaccine Targeting the Vaccinia Virus L1, A27, B5, and A33 Proteins Protects Rabbits against Lethal Rabbitpox Virus Aerosol Challenge.J Virol. 2022 Feb 9;96(3):e0150421. doi: 10.1128/JVI.01504-21. Epub 2021 Dec 1. J Virol. 2022. PMID: 34851148 Free PMC article.
-
Third-generation smallpox vaccines: challenges in the absence of clinical smallpox.Future Microbiol. 2010 Sep;5(9):1367-82. doi: 10.2217/fmb.10.98. Future Microbiol. 2010. PMID: 20860482 Review.
-
Rabbitpox: a model of airborne transmission of smallpox.J Gen Virol. 2011 Jan;92(Pt 1):31-5. doi: 10.1099/vir.0.026237-0. Epub 2010 Oct 21. J Gen Virol. 2011. PMID: 20965981 Review.
Cited by
-
Monoclonal Antibodies in Light of Mpox Outbreak: Current Research, Therapeutic Targets, and Animal Models.Antibodies (Basel). 2025 Feb 26;14(1):20. doi: 10.3390/antib14010020. Antibodies (Basel). 2025. PMID: 40136469 Free PMC article. Review.
-
Discovery of a heparan sulfate binding domain in monkeypox virus H3 as an anti-poxviral drug target combining AI and MD simulations.Elife. 2025 Jan 16;13:RP100545. doi: 10.7554/eLife.100545. Elife. 2025. PMID: 39817728 Free PMC article.
-
Challenges and Achievements in Prevention and Treatment of Smallpox.Vaccines (Basel). 2018 Jan 29;6(1):8. doi: 10.3390/vaccines6010008. Vaccines (Basel). 2018. PMID: 29382130 Free PMC article. Review.
-
Modified Vaccinia Ankara Virus Vaccination Provides Long-Term Protection against Nasal Rabbitpox Virus Challenge.Clin Vaccine Immunol. 2016 Jul 5;23(7):648-51. doi: 10.1128/CVI.00216-16. Print 2016 Jul. Clin Vaccine Immunol. 2016. PMID: 27146001 Free PMC article.
-
Lipid nanoparticle delivery of unmodified mRNAs encoding multiple monoclonal antibodies targeting poxviruses in rabbits.Mol Ther Nucleic Acids. 2022 May 10;28:847-858. doi: 10.1016/j.omtn.2022.05.025. eCollection 2022 Jun 14. Mol Ther Nucleic Acids. 2022. PMID: 35664703 Free PMC article.
References
-
- Henderson DA, Inglesby TV, Bartlett JG, Ascher MS, Eitzen E, et al. (1999) Smallpox as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. Jama 281: 2127–2137. - PubMed
-
- Wittek R (2006) Vaccinia immune globulin: current policies, preparedness, and product safety and efficacy. Int J Infect Dis 10: 193–201. - PubMed
-
- Fulginiti VA, Papier A, Lane JM, Neff JM, Henderson DA (2003) Smallpox vaccination: a review, part I. Background, vaccination technique, normal vaccination and revaccination, and expected normal reactions. Clin Infect Dis 37: 241–250. - PubMed
-
- Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi I (2001) Smallpox and its Eradication. 4.
-
- Huhn GD, Bauer AM, Yorita K, Graham MB, Sejvar J, et al. (2005) Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin Infect Dis 41: 1742–1751. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

