Endosomal maturation, Rab7 GTPase and phosphoinositides in African swine fever virus entry
- PMID: 23133661
- PMCID: PMC3486801
- DOI: 10.1371/journal.pone.0048853
Endosomal maturation, Rab7 GTPase and phosphoinositides in African swine fever virus entry
Abstract
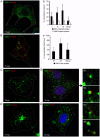
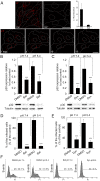
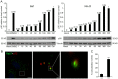
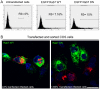
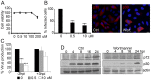
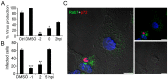
Here we analyzed the dependence of African swine fever virus (ASFV) infection on the integrity of the endosomal pathway. Using confocal immunofluorescence with antibodies against viral capsid proteins, we found colocalization of incoming viral particles with early endosomes (EE) during the first minutes of infection. Conversely, viral capsid protein was not detected in acidic late endosomal compartments, multivesicular bodies (MVBs), late endosomes (LEs) or lysosomes (LY). Using an antibody against a viral inner core protein, we found colocalization of viral cores with late compartments from 30 to 60 minutes postinfection. The absence of capsid protein staining in LEs and LYs suggested that virus desencapsidation would take place at the acid pH of these organelles. In fact, inhibitors of intraluminal acidification of endosomes caused retention of viral capsid staining virions in Rab7 expressing endosomes and more importantly, severely impaired subsequent viral protein production. Endosomal acidification in the first hour after virus entry was essential for successful infection but not thereafter. In addition, altering the balance of phosphoinositides (PIs) which are responsible of the maintenance of the endocytic pathway impaired ASFV infection. Early infection steps were dependent on the production of phosphatidylinositol 3-phosphate (PtdIns3P) which is involved in EE maturation and multivesicular body (MVB) biogenesis and on the interconversion of PtdIns3P to phosphatidylinositol 3, 5-biphosphate (PtdIns(3,5)P(2)). Likewise, GTPase Rab7 activity should remain intact, as well as processes related to LE compartment physiology, which are crucial during early infection. Our data demonstrate that the EE and LE compartments and the integrity of the endosomal maturation pathway orchestrated by Rab proteins and PIs play a central role during early stages of ASFV infection.
Conflict of interest statement
Figures
Similar articles
-
New insights into the role of endosomal proteins for African swine fever virus infection.PLoS Pathog. 2022 Jan 26;18(1):e1009784. doi: 10.1371/journal.ppat.1009784. eCollection 2022 Jan. PLoS Pathog. 2022. PMID: 35081156 Free PMC article.
-
African Swine Fever Virus Undergoes Outer Envelope Disruption, Capsid Disassembly and Inner Envelope Fusion before Core Release from Multivesicular Endosomes.PLoS Pathog. 2016 Apr 25;12(4):e1005595. doi: 10.1371/journal.ppat.1005595. eCollection 2016 Apr. PLoS Pathog. 2016. PMID: 27110717 Free PMC article.
-
Cholesterol Flux Is Required for Endosomal Progression of African Swine Fever Virions during the Initial Establishment of Infection.J Virol. 2015 Nov 25;90(3):1534-43. doi: 10.1128/JVI.02694-15. Print 2016 Feb 1. J Virol. 2015. PMID: 26608317 Free PMC article.
-
Mechanisms of Entry and Endosomal Pathway of African Swine Fever Virus.Vaccines (Basel). 2017 Nov 8;5(4):42. doi: 10.3390/vaccines5040042. Vaccines (Basel). 2017. PMID: 29117102 Free PMC article. Review.
-
African Swine Fever Virus Gets Undressed: New Insights on the Entry Pathway.J Virol. 2017 Jan 31;91(4):e01906-16. doi: 10.1128/JVI.01906-16. Print 2017 Feb 15. J Virol. 2017. PMID: 27974557 Free PMC article. Review.
Cited by
-
ASFV infection induces lipid metabolic disturbances and promotes viral replication.Front Microbiol. 2025 Jan 7;15:1532678. doi: 10.3389/fmicb.2024.1532678. eCollection 2024. Front Microbiol. 2025. PMID: 39872814 Free PMC article.
-
Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)1.Autophagy. 2021 Jan;17(1):1-382. doi: 10.1080/15548627.2020.1797280. Epub 2021 Feb 8. Autophagy. 2021. PMID: 33634751 Free PMC article.
-
African Swine Fever Virus Exhibits Distinct Replication Defects in Different Cell Types.Viruses. 2022 Nov 26;14(12):2642. doi: 10.3390/v14122642. Viruses. 2022. PMID: 36560646 Free PMC article.
-
Feline herpesvirus 1 (FHV-1) enters the cell by receptor-mediated endocytosis.J Virol. 2023 Aug 31;97(8):e0068123. doi: 10.1128/jvi.00681-23. Epub 2023 Jul 26. J Virol. 2023. PMID: 37493545 Free PMC article.
-
The Potential of Disabled Infectious Single Cycle (DISC) Virus Platforms for Next Generation African Swine Fever Vaccine Development.Transbound Emerg Dis. 2025 Jul 14;2025:8573171. doi: 10.1155/tbed/8573171. eCollection 2025. Transbound Emerg Dis. 2025. PMID: 40692871 Free PMC article. Review.
References
-
- Dixon L, Costa JV, Escribano JM, Rock DL, Vinuela E, et al.. (2000) Asfarviridae. New York: Academic Press. 159–165 p.
-
- Breese SS Jr, DeBoer CJ (1966) Electron microscope observations of African swine fever virus in tissue culture cells. Virology 28: 420–428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

