Fibroblast growth factor 10-fibroblast growth factor receptor 2b mediated signaling is not required for adult glandular stomach homeostasis
- PMID: 23133671
- PMCID: PMC3486796
- DOI: 10.1371/journal.pone.0049127
Fibroblast growth factor 10-fibroblast growth factor receptor 2b mediated signaling is not required for adult glandular stomach homeostasis
Abstract
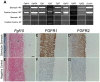
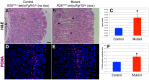
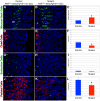
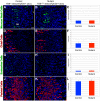
The signaling pathways that are essential for gastric organogenesis have been studied in some detail; however, those that regulate the maintenance of the gastric epithelium during adult homeostasis remain unclear. In this study, we investigated the role of Fibroblast growth factor 10 (FGF10) and its main receptor, Fibroblast growth factor receptor 2b (FGFR2b), in adult glandular stomach homeostasis. We first showed that mouse adult glandular stomach expressed Fgf10, its receptors, Fgfr1b and Fgfr2b, and most of the other FGFR2b ligands (Fgf1, Fgf7, Fgf22) except for Fgf3 and Fgf20. Fgf10 expression was mesenchymal whereas FGFR1 and FGFR2 expression were mostly epithelial. Studying double transgenic mice that allow inducible overexpression of Fgf10 in adult mice, we showed that Fgf10 overexpression in normal adult glandular stomach increased epithelial proliferation, drove mucous neck cell differentiation, and reduced parietal and chief cell differentiation. Although a similar phenotype can be associated with the development of metaplasia, we found that Fgf10 overexpression for a short duration does not cause metaplasia. Finally, investigating double transgenic mice that allow the expression of a soluble form of Fgfr2b, FGF10's main receptor, which acts as a dominant negative, we found no significant changes in gastric epithelial proliferation or differentiation in the mutants. Our work provides evidence, for the first time, that the FGF10-FGFR2b signaling pathway is not required for epithelial proliferation and differentiation during adult glandular stomach homeostasis.
Conflict of interest statement
Figures


References
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, et al. (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127: 2893–2917. - PubMed
-
- Kamangar F, Dores GM, Anderson WF (2006) Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 24: 2137–2150. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

