The single T65S mutation generates brighter cyan fluorescent proteins with increased photostability and pH insensitivity
- PMID: 23133673
- PMCID: PMC3487735
- DOI: 10.1371/journal.pone.0049149
The single T65S mutation generates brighter cyan fluorescent proteins with increased photostability and pH insensitivity
Abstract
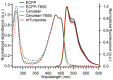
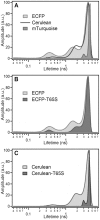
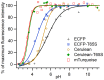
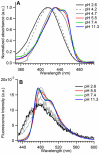
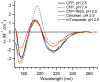
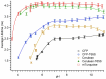
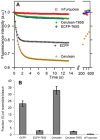
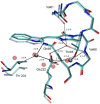
Cyan fluorescent proteins (CFP) derived from Aequorea victoria GFP, carrying a tryptophan-based chromophore, are widely used as FRET donors in live cell fluorescence imaging experiments. Recently, several CFP variants with near-ultimate photophysical performances were obtained through a mix of site-directed and large scale random mutagenesis. To understand the structural bases of these improvements, we have studied more specifically the consequences of the single-site T65S mutation. We find that all CFP variants carrying the T65S mutation not only display an increased fluorescence quantum yield and a simpler fluorescence emission decay, but also show an improved pH stability and strongly reduced reversible photoswitching reactions. Most prominently, the Cerulean-T65S variant reaches performances nearly equivalent to those of mTurquoise, with QY = 0.84, an almost pure single exponential fluorescence decay and an outstanding stability in the acid pH range (pK(1/2) = 3.6). From the detailed examination of crystallographic structures of different CFPs and GFPs, we conclude that these improvements stem from a shift in the thermodynamic balance between two well defined configurations of the residue 65 hydroxyl. These two configurations differ in their relative stabilization of a rigid chromophore, as well as in relaying the effects of Glu222 protonation at acid pHs. Our results suggest a simple method to greatly improve numerous FRET reporters used in cell imaging, and bring novel insights into the general structure-photophysics relationships of fluorescent proteins.
Conflict of interest statement
Figures
References
-
- Zhang J, Allen MD (2007) FRET-based biosensors for protein kinases: illuminating the kinome. Molecular Biosyst 3: 759–765. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

