CT694 and pgp3 as serological tools for monitoring trachoma programs
- PMID: 23133684
- PMCID: PMC3486877
- DOI: 10.1371/journal.pntd.0001873
CT694 and pgp3 as serological tools for monitoring trachoma programs
Abstract
Background: Defining endpoints for trachoma programs can be a challenge as clinical signs of infection may persist in the absence of detectable bacteria. Antibody-based tests may provide an alternative testing strategy for surveillance during terminal phases of the program. Antibody-based assays, in particular ELISAs, have been shown to be useful to document C. trachomatis genital infections, but have not been explored extensively for ocular C. trachomatis infections.
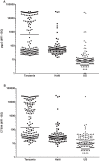
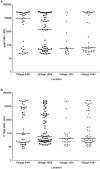
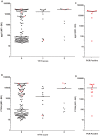
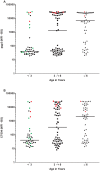
Methodology/principal findings: An antibody-based multiplex assay was used to test two C. trachomatis antigens, pgp3 and CT694, for detection of trachoma antibodies in bloodspots from Tanzanian children (n = 160) collected after multiple rounds of mass azithromycin treatment. Using samples from C. trachomatis-positive (by PCR) children from Tanzania (n = 11) and control sera from a non-endemic group of U.S. children (n = 122), IgG responses to both pgp3 and CT694 were determined to be 91% sensitive and 98% specific. Antibody responses of Tanzanian children were analyzed with regard to clinical trachoma, PCR positivity, and age. In general, children with more intense ocular pathology (TF/TI = 2 or most severe) had a higher median antibody response to pgp3 (p = 0.0041) and CT694 (p = 0.0282) than those with normal exams (TF/TI = 0). However, 44% of children with no ocular pathology tested positive for antibody, suggesting prior infection. The median titer of antibody responses for children less than three years of age was significantly lower than those of older children. (p<0.0001 for both antigens).
Conclusions/significance: The antibody-based multiplex assay is a sensitive and specific additional tool for evaluating trachoma transmission. The assay can also be expanded to include antigens representing different diseases, allowing for a robust assay for monitoring across NTD programs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Community-level chlamydial serology for assessing trachoma elimination in trachoma-endemic Niger.PLoS Negl Trop Dis. 2019 Jan 28;13(1):e0007127. doi: 10.1371/journal.pntd.0007127. eCollection 2019 Jan. PLoS Negl Trop Dis. 2019. PMID: 30689671 Free PMC article. Clinical Trial.
-
Serology for trachoma surveillance after cessation of mass drug administration.PLoS Negl Trop Dis. 2015 Feb 25;9(2):e0003555. doi: 10.1371/journal.pntd.0003555. eCollection 2015 Feb. PLoS Negl Trop Dis. 2015. PMID: 25714363 Free PMC article.
-
Seroepidemiology of trachoma in a low prevalence region receiving annual mass azithromycin distribution in Maradi, Niger.PLoS Negl Trop Dis. 2024 Dec 9;18(12):e0012727. doi: 10.1371/journal.pntd.0012727. eCollection 2024 Dec. PLoS Negl Trop Dis. 2024. PMID: 39652587 Free PMC article.
-
Prevalence of ocular Chlamydia trachomatis infection and antibodies within districts persistently endemic for trachoma, Amhara, Ethiopia.PLoS Negl Trop Dis. 2025 Mar 11;19(3):e0012900. doi: 10.1371/journal.pntd.0012900. eCollection 2025 Mar. PLoS Negl Trop Dis. 2025. PMID: 40067808 Free PMC article.
-
Chlamydia trachomatis: serological diagnosis.Infection. 1982;10 Suppl 1:S25-31. doi: 10.1007/BF01640711. Infection. 1982. PMID: 7044980 Review.
Cited by
-
The effect of Mass Drug Administration for trachoma on antibodies to Chlamydia trachomatis pgp3 in children.Sci Rep. 2020 Sep 16;10(1):15225. doi: 10.1038/s41598-020-71833-x. Sci Rep. 2020. PMID: 32938957 Free PMC article.
-
Chlamydial Plasmid-Dependent Pathogenicity.Trends Microbiol. 2017 Feb;25(2):141-152. doi: 10.1016/j.tim.2016.09.006. Epub 2016 Oct 3. Trends Microbiol. 2017. PMID: 27712952 Free PMC article. Review.
-
Discovery of Human-Specific Immunodominant Chlamydia trachomatis B Cell Epitopes.mSphere. 2018 Aug 1;3(4):e00246-18. doi: 10.1128/mSphere.00246-18. mSphere. 2018. PMID: 30068558 Free PMC article.
-
Azithromycin Reduction to Reach Elimination of Trachoma (ARRET): study protocol for a cluster randomized trial of stopping mass azithromycin distribution for trachoma.BMC Ophthalmol. 2021 Jan 6;21(1):15. doi: 10.1186/s12886-020-01776-4. BMC Ophthalmol. 2021. PMID: 33407263 Free PMC article.
-
Determining seropositivity-A review of approaches to define population seroprevalence when using multiplex bead assays to assess burden of tropical diseases.PLoS Negl Trop Dis. 2021 Jun 28;15(6):e0009457. doi: 10.1371/journal.pntd.0009457. eCollection 2021 Jun. PLoS Negl Trop Dis. 2021. PMID: 34181665 Free PMC article. Review.
References
-
- Mariotti SP, Pascolini D, Rose-Nussbaumer J (2009) Trachoma: global magnitude of a preventable cause of blindness. Br J Ophthalmol 93: 563–568. - PubMed
-
- Frick KD, Basilion EV, Hanson CL, Colchero MA (2003) Estimating the burden and economic impact of trachomatous visual loss. Ophthalmic Epidemiol 10: 121–132. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

