Accuracy of a dual path platform (DPP) assay for the rapid point-of-care diagnosis of human leptospirosis
- PMID: 23133686
- PMCID: PMC3486890
- DOI: 10.1371/journal.pntd.0001878
Accuracy of a dual path platform (DPP) assay for the rapid point-of-care diagnosis of human leptospirosis
Abstract
Background: Diagnosis of leptospirosis by the gold standard serologic assay, the microscopic agglutination test (MAT), requires paired sera and is not widely available. We developed a rapid assay using immunodominant Leptospira immunoglobulin-like (Lig) proteins in a Dual Path Platform (DPP). This study aimed to evaluate the assay's diagnostic performance in the setting of urban transmission.
Methodology: We determined test sensitivity using 446 acute and convalescent sera from MAT-confirmed case-patients with severe or mild leptospirosis in Brazil. We assessed test specificity using 677 sera from the following groups: healthy residents of a Brazilian slum with endemic transmission, febrile outpatients from the same slum, healthy blood donors, and patients with dengue, hepatitis A, and syphilis. Three operators independently interpreted visual results without knowing specimen status.
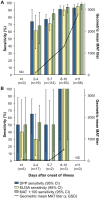
Results: The overall sensitivity for paired sera was 100% and 73% for severe and mild disease, respectively. In the acute phase, the assay achieved a sensitivity of 85% and 64% for severe and mild leptospirosis, respectively. Within seven days of illness onset, the assay achieved a sensitivity of 77% for severe disease and 60% for mild leptospirosis. Sensitivity of the DPP assay was similar to that for IgM-ELISA and increased with both duration of symptoms (chi-square regression P = 0.002) and agglutinating titer (Spearman ρ = 0.24, P<0.001). Specificity was ≥93% for dengue, hepatitis A, syphilis, febrile outpatients, and blood donors, while it was 86% for healthy slum residents. Inter-operator agreement ranged from very good to excellent (kappa: 0.82-0.94) and test-to-test reproducibility was also high (kappa: 0.89).
Conclusions: The DPP assay performed acceptably well for diagnosis of severe acute clinical leptospirosis and can be easily implemented in hospitals and health posts where leptospirosis is a major public health problem. However, test accuracy may need improvement for mild disease and early stage leptospirosis, particularly in regions with high transmission.
Conflict of interest statement
The authors have read the journal's policy and have the following conflicts: AIK and MGR are holders of the U.S. patent 8,124,110 for the proteins with repetitive bacterial-Ig-like (Big) domains present in Leptospira species used as antigens in the DPP assay. JE holds U.S. patent 7,879,597 for the dual path immunoassay device (DPP) evaluated in this study. JE, KPL, and RG are employees of Chembio Diagnostic Systems, Inc., which developed the DPP assay for leptospirosis and has transferred the DPP technology to the Brazilian Ministry of Health.
Figures

Similar articles
-
Prospective evaluation of accuracy and clinical utility of the Dual Path Platform (DPP) assay for the point-of-care diagnosis of leptospirosis in hospitalized patients.PLoS Negl Trop Dis. 2018 Feb 20;12(2):e0006285. doi: 10.1371/journal.pntd.0006285. eCollection 2018 Feb. PLoS Negl Trop Dis. 2018. PMID: 29462146 Free PMC article.
-
Diagnosis of Leptospirosis: Comparison between Microscopic Agglutination Test, IgM-ELISA and IgM Rapid Immunochromatography Test.PLoS One. 2015 Jun 18;10(6):e0129236. doi: 10.1371/journal.pone.0129236. eCollection 2015. PLoS One. 2015. PMID: 26086800 Free PMC article.
-
Detection of human leptospirosis as a cause of acute fever by capture ELISA using a Leptospira interrogans serovar Copenhageni (M20) derived antigen.BMC Infect Dis. 2013 Sep 20;13:438. doi: 10.1186/1471-2334-13-438. BMC Infect Dis. 2013. PMID: 24053555 Free PMC article.
-
Nucleic acid and antigen detection tests for leptospirosis.Cochrane Database Syst Rev. 2019 Aug 1;8(8):CD011871. doi: 10.1002/14651858.CD011871.pub2. Cochrane Database Syst Rev. 2019. PMID: 31425612 Free PMC article.
-
Diagnostic accuracy of leptospirosis whole-cell lateral flow assays: a systematic review and meta-analysis.Clin Microbiol Infect. 2019 Apr;25(4):437-444. doi: 10.1016/j.cmi.2018.11.014. Epub 2018 Nov 23. Clin Microbiol Infect. 2019. PMID: 30472422 Free PMC article.
Cited by
-
Serodiagnosis of equine leptospirosis by enzyme-linked immunosorbent assay using four recombinant protein markers.Clin Vaccine Immunol. 2014 Apr;21(4):478-83. doi: 10.1128/CVI.00649-13. Epub 2014 Jan 22. Clin Vaccine Immunol. 2014. PMID: 24451330 Free PMC article.
-
Rainfall and other meteorological factors as drivers of urban transmission of leptospirosis.PLoS Negl Trop Dis. 2022 Apr 11;16(4):e0007507. doi: 10.1371/journal.pntd.0007507. eCollection 2022 Apr. PLoS Negl Trop Dis. 2022. PMID: 35404948 Free PMC article.
-
Recombinant antigens rLipL21, rLoa22, rLipL32 and rLigACon4-8 for serological diagnosis of leptospirosis by enzyme-linked immunosorbent assays in dogs.PLoS One. 2014 Dec 19;9(12):e111367. doi: 10.1371/journal.pone.0111367. eCollection 2014. PLoS One. 2014. PMID: 25526513 Free PMC article.
-
Production of recombinant electron transfer flavoprotein beta subunit protein and its application in a lateral flow assay for early diagnosis of leptospirosis.Med Microbiol Immunol. 2025 Feb 19;214(1):12. doi: 10.1007/s00430-025-00822-6. Med Microbiol Immunol. 2025. PMID: 39969576
-
Identification of seroreactive proteins of Leptospira interrogans serovar copenhageni using a high-density protein microarray approach.PLoS Negl Trop Dis. 2013 Oct 17;7(10):e2499. doi: 10.1371/journal.pntd.0002499. eCollection 2013. PLoS Negl Trop Dis. 2013. PMID: 24147173 Free PMC article.
References
-
- WHO (1999) Leptospirosis worldwide. Weekly Epidemiological Record 74: 237–242. - PubMed
-
- Pappas G, Papadimitriou P, Siozopoulou V, Christou L, Akritidis N (2008) The globalization of leptospirosis: worldwide incidence trends. Int J Infect Dis 12: 351–357. - PubMed
-
- Sarkar U, Nascimento SF, Barbosa R, Martins R, Nuevo H, et al. (2002) Population-based case-control investigation of risk factors for leptospirosis during an urban epidemic. Am J Trop Med Hyg 66: 605–610. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

