Current imaging strategies in rheumatoid arthritis
- PMID: 23133812
- PMCID: PMC3477730
Current imaging strategies in rheumatoid arthritis
Abstract
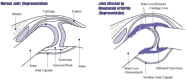
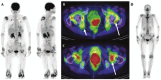
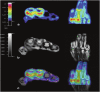
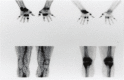
As remission has now become a realistic therapeutic goal in the clinical management of RA due to the introduction and widespread adoption of biologic agents, there is a greater need for earlier diagnoses and objective methods for evaluating disease activity and response to treatment. In this capacity, advanced imaging strategies are assuming an expansive clinical role, particularly as they take advantage of newer imaging technologies and the shift toward imaging at the molecular level. Molecular imaging utilizes target-specific probes to non-invasively visualize molecular, cellular, and physiological perturbations in response to the underlying pathology. Probes for nuclear and MR imaging have been and are being developed that react with discrete aspects of inflammatory and destructive pathways specific to RA. These probes in addition to new MR sequences and contrast agents have the potential to provide an earlier and more reliable assessment of clinical outcome, disease activity, severity, and location, and therapeutic response. Furthermore, these imaging strategies may enable a more fundamental understanding of critical pathophysiological processes and the advent of new molecular therapies. This review will discuss these advances in both nuclear medicine and MRI strategies for imaging RA with a particular emphasis on molecular imaging.
Keywords: Molecular imaging; magnetic resonance imaging; nuclear imaging; rheumatoid arthritis.
Figures
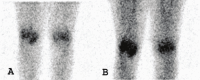

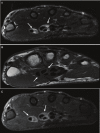
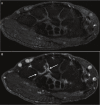
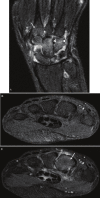
References
-
- Gabriel SE. The epidemiology of rheumatoid arthritis. Rheum Dis Clin North Am. 2001;27:269–281. - PubMed
-
- Pincus T, Callahan LF, Sale WG, Brooks AL, Payne LE, Vaughn WK. Severe functional declines, work disability, and increased mortality in seventy-five rheumatoid arthritis patients studied over nine years. Arthritis Rheum. 1984;27:864–872. - PubMed
-
- Wolfe F, Mitchell DM, Sibley JT, Fries JF, Bloch DA, Williams CA, Spitz PW, Haga M, Kleinheksel SM, Cathey MA. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37:481–494. - PubMed
-
- Choy EH, Kingsley GH, Panayi GS. Monoclonal antibody therapy in rheumatoid arthritis. Br J Rheumatol. 1998;37:484–490. - PubMed
-
- Fan PT, Leong KH. The use of biological agents in the treatment of rheumatoid arthritis. Ann Acad Med Singapore. 2007;36:128–134. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
