[(125)I]FIAU imaging in a preclinical model of lung infection: quantification of bacterial load
- PMID: 23133816
- PMCID: PMC3477740
[(125)I]FIAU imaging in a preclinical model of lung infection: quantification of bacterial load
Abstract
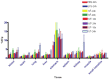
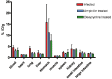
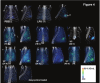
2'-Fluoro-2'-deoxy-1β-D-arabinofuranosyl-5-[(125)I]iodouracil ([(125)I]FIAU), a substrate for the thymidine kinase (TK) present in most bacteria, has been used as an imaging agent for single photon emission computed tomography (SPECT) in an experimental model of lung infection. Using SPECT-CT we show that [(125)I]FIAU is specific for bacterial infection rather than sterile inflammation. We report [(125)I]FIAU lung uptake values of 1.26 ± 0.20 percent injected dose per gram (%ID/g) in normal controls, 1.69 ± 0.32 %ID/g in lung inflammation and up to 7.14 ± 1.09 %ID/g in lung infection in ex vivo biodistribution studies at 24 h after intranasal administration of bacteria. Images of [(125)I]FIAU signal within lung can be used to estimate the number of bacteria present, with a limit of detection of 10(9) colony forming units per mL on the X-SPECT scanner. [(125)I]FIAU-Based bacterial imaging may be useful in preclinical models to facilitate the development of new antibiotics, particularly in cases where a corresponding human trial is planned.
Keywords: Inflammation; PET; SPECT; molecular imaging; nucleoside; thymidine kinase.
Figures



Similar articles
-
Quantitative kinetics of [124I]FIAU in cat and man.J Nucl Med. 2001 Mar;42(3):467-75. J Nucl Med. 2001. PMID: 11337525
-
Monitoring bone marrow stem cells with a reporter gene system in experimental middle cerebral artery occlusion rat models.J Nucl Med. 2013 Jun;54(6):984-9. doi: 10.2967/jnumed.112.109280. Epub 2013 Mar 27. J Nucl Med. 2013. PMID: 23536224
-
[Myocardial single photon emission tomography imaging of reporter gene expression in rabbits].Zhonghua Xin Xue Guan Bing Za Zhi. 2009 Jun;37(6):548-53. Zhonghua Xin Xue Guan Bing Za Zhi. 2009. PMID: 19927639 Chinese.
-
Herpes simplex virus thymidine kinase as a marker/reporter gene for PET imaging of gene therapy.Q J Nucl Med. 1999 Jun;43(2):163-9. Q J Nucl Med. 1999. PMID: 10429512 Review.
-
(68)Ga-radiopharmaceuticals for PET imaging of infection and inflammation.Recent Results Cancer Res. 2013;194:189-219. doi: 10.1007/978-3-642-27994-2_11. Recent Results Cancer Res. 2013. PMID: 22918761 Review.
Cited by
-
Engineered live bacteria as disease detection and diagnosis tools.J Biol Eng. 2023 Oct 24;17(1):65. doi: 10.1186/s13036-023-00379-z. J Biol Eng. 2023. PMID: 37875910 Free PMC article. Review.
-
Molecular imaging of pulmonary diseases.Respir Res. 2018 Jan 24;19(1):17. doi: 10.1186/s12931-018-0716-0. Respir Res. 2018. PMID: 29368614 Free PMC article. Review.
-
Evaluation of 1-(2-deoxy-2-fluoro-1-D-arabinofuranosyl)-5-iodouracil (FIAU) as an ex vivo bacterial detection agent.World J Microbiol Biotechnol. 2014 Nov;30(11):3003-10. doi: 10.1007/s11274-014-1709-x. Epub 2014 Aug 2. World J Microbiol Biotechnol. 2014. PMID: 25085723
-
Recent Progress in the Molecular Imaging of Tumor-Treating Bacteria.Nucl Med Mol Imaging. 2021 Feb;55(1):7-14. doi: 10.1007/s13139-021-00689-4. Epub 2021 Jan 31. Nucl Med Mol Imaging. 2021. PMID: 33643484 Free PMC article. Review.
-
Pathogen-Specific Bacterial Imaging in Nuclear Medicine.Semin Nucl Med. 2018 Mar;48(2):182-194. doi: 10.1053/j.semnuclmed.2017.11.003. Epub 2017 Dec 14. Semin Nucl Med. 2018. PMID: 29452620 Free PMC article. Review.
References
-
- Jarvis WR. Prevention and control of methicillin-resistant Staphylococcus aureus: dealing with reality, resistance, and resistance to reality. Clin Infect Dis. 2010;50:218–220. - PubMed
-
- Subha A, Ananthan S. Extended spectrum beta lactamase (ESBL) mediated resistance to third generation cephalosporins among Klebsiella pneumoniae in Chennai. Indian J Med Microbiol. 2002;20:92–95. - PubMed
-
- Samonis G, Bafaloukos D. Fungal infections in cancer patients: an escalating problem. In Vivo. 1992;6:183–193. - PubMed
-
- Gasink LB, Blumberg EA. Bacterial and mycobacterial pneumonia in transplant recipients. Clin Chest Med. 2005;26:647–659. vii. - PubMed
-
- Vinh DC, Embil JM. Device-related infections: a review. J Long Term Eff Med Implants. 2005;15:467–488. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
