Consumer involvement in topic and outcome selection in the development of clinical practice guidelines
- PMID: 23134217
- PMCID: PMC5060636
- DOI: 10.1111/j.1369-7625.2011.00676.x
Consumer involvement in topic and outcome selection in the development of clinical practice guidelines
Abstract
Background: Consumer involvement in guideline development is advocated, but minimal participation, such as a nominated consumer representative on a guideline working group, can inhibit their decision-making power and contribution. Little is known about how to involve consumers more effectively in guideline development.
Objective: To describe a targeted approach for involving consumers actively in guideline development, by focusing on topic and outcome selection, and to discuss the impact on content and structure of the final guideline.
Design: Descriptive study.
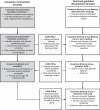
Setting and participants: Patients and carers (n = 24) from a tertiary hospital in Sydney attended three structured peer-facilitated workshops to complete group-based exercises on topic and outcome selection for guidelines for early stage chronic kidney disease. These workshops were run in parallel with the guideline-writing group. For each exercise, participants formed small groups and facilitated their own discussion, recorded their responses and presented them to the wider group. The topics and outcomes identified were fed back to the guideline writers.
Results: The participants actively engaged in the workshop discussions and articulated topics and outcomes they perceived should be included in clinical guidelines. Four main changes to guideline-related outputs were observed. A new guideline subtopic was introduced, guidelines were consumer-endorsed, guideline recommendations and suggestions for clinical care were augmented with consumer-focused issues, and plain English guidelines were developed.
Conclusions: Consumer workshops in parallel and feeding into guideline development can be a feasible and effective approach for active consumer contribution. This process can inform the development of both consumer-focused guidelines for clinicians and specific versions for consumers.
© 2011 Blackwell Publishing Ltd.
Figures
References
-
- Krahn M, Naglie G. The next step in guideline development: incorporating patient preferences. Journal of the American Medical Association, 2008; 300: 436–438. - PubMed
-
- Boivin A, Green J, van derMeulenJ, Legare F, Nolte E. Why consider patients’ preferences? A discourse analysis of clinical practice guideline developers Medical Care, 2009; 47: 908–915. - PubMed
-
- Boivin A, Currie K, Fervers B et al. Patient and public involvement in clinical guidelines: international experiences and future perspectives. Quality and Safety in Health Care. 2010; 19: 1–4. - PubMed
-
- Bastian H. Raising the standard: practice guidelines and consumer participation. International Journal for Quality in Health Care, 1996; 8: 485–490. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical