Progression of RAS-mutant leukemia during RAF inhibitor treatment
- PMID: 23134356
- PMCID: PMC3627494
- DOI: 10.1056/NEJMoa1208958
Progression of RAS-mutant leukemia during RAF inhibitor treatment
Abstract
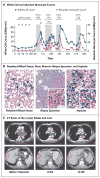
Vemurafenib, a selective RAF inhibitor, extends survival among patients with BRAF V600E-mutant melanoma. Vemurafenib inhibits ERK signaling in BRAF V600E-mutant cells but activates ERK signaling in BRAF wild-type cells. This paradoxical activation of ERK signaling is the mechanistic basis for the development of RAS-mutant squamous-cell skin cancers in patients treated with RAF inhibitors. We report the accelerated growth of a previously unsuspected RAS-mutant leukemia in a patient with melanoma who was receiving vemurafenib. Exposure to vemurafenib induced hyperactivation of ERK signaling and proliferation of the leukemic cell population, an effect that was reversed on drug withdrawal.
Figures

References
-
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. - PubMed
-
- Gorden A, Osman I, Gai W, et al. Analysis of BRAF and N-RAS mutations in metastatic melanoma tissues. Cancer Res. 2003;63:3955–7. - PubMed
-
- Menzies AM, Haydu LE, Visintin L, et al. Distinguishing clinicopathologic features of patients with V600E and V600K BRAF-mutant metastatic melanoma. Clin Cancer Res. 2012;18:3242–9. - PubMed
-
- Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–67. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
