Immunohistological and electrophysiological evidence that N-acetylaspartylglutamate is a co-transmitter at the vertebrate neuromuscular junction
- PMID: 23134476
- PMCID: PMC3538924
- DOI: 10.1111/ejn.12027
Immunohistological and electrophysiological evidence that N-acetylaspartylglutamate is a co-transmitter at the vertebrate neuromuscular junction
Abstract
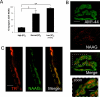
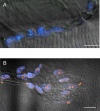
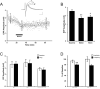
Immunohistochemical studies previously revealed the presence of the peptide transmitter N-acetylaspartylglutamate (NAAG) in spinal motor neurons, axons and presumptive neuromuscular junctions (NMJs). At synapses in the central nervous system, NAAG has been shown to activate the type 3 metabotropic glutamate receptor (mGluR3) and is inactivated by an extracellular peptidase, glutamate carboxypeptidase II. The present study tested the hypothesis that NAAG meets the criteria for classification as a co-transmitter at the vertebrate NMJ. Confocal microscopy confirmed the presence of NAAG immunoreactivity and extended the resolution of the peptide's location in the lizard (Anolis carolinensis) NMJ. NAAG was localised to a presynaptic region immediately adjacent to postsynaptic acetylcholine receptors. NAAG was depleted by potassium-induced depolarisation and by electrical stimulation of motor axons. The NAAG receptor, mGluR3, was localised to the presynaptic terminal consistent with NAAG's demonstrated role as a regulator of synaptic release at central synapses. In contrast, glutamate receptors, type 2 metabotropic glutamate receptor (mGluR2) and N-methyl-d-aspartate, were closely associated with acetylcholine receptors in the postsynaptic membrane. Glutamate carboxypeptidase II, the NAAG-inactivating enzyme, was identified exclusively in perisynaptic glial cells. This localisation was confirmed by the loss of immunoreactivity when these cells were selectively eliminated. Finally, electrophysiological studies showed that exogenous NAAG inhibited evoked neurotransmitter release by activating a group II metabotropic glutamate receptor (mGluR2 or mGluR3). Collectively, these data support the conclusion that NAAG is a co-transmitter at the vertebrate NMJ.
© 2012 Federation of European Neuroscience Societies and Blackwell Publishing Ltd.
Figures




Similar articles
-
beta-NAAG rescues LTP from blockade by NAAG in rat dentate gyrus via the type 3 metabotropic glutamate receptor.J Neurophysiol. 2001 Mar;85(3):1097-106. doi: 10.1152/jn.2001.85.3.1097. J Neurophysiol. 2001. PMID: 11247980
-
Endogenous N-acetylaspartylglutamate (NAAG) inhibits synaptic plasticity/transmission in the amygdala in a mouse inflammatory pain model.Mol Pain. 2010 Sep 22;6:60. doi: 10.1186/1744-8069-6-60. Mol Pain. 2010. PMID: 20860833 Free PMC article.
-
N-acetylaspartylglutamate activates cyclic AMP-coupled metabotropic glutamate receptors in cerebellar astrocytes.Glia. 1998 Oct;24(2):172-9. doi: 10.1002/(sici)1098-1136(199810)24:2<172::aid-glia2>3.0.co;2-6. Glia. 1998. PMID: 9728763
-
N-Acetylaspartylglutamate: the most abundant peptide neurotransmitter in the mammalian central nervous system.J Neurochem. 2000 Aug;75(2):443-52. doi: 10.1046/j.1471-4159.2000.0750443.x. J Neurochem. 2000. PMID: 10899918 Review.
-
NAAG, NMDA receptor and psychosis.Curr Med Chem. 2012;19(9):1360-4. doi: 10.2174/092986712799462685. Curr Med Chem. 2012. PMID: 22304714 Free PMC article. Review.
Cited by
-
Inhibition of glutamate-carboxypeptidase-II in dorsolateral prefrontal cortex: potential therapeutic target for neuroinflammatory cognitive disorders.Mol Psychiatry. 2022 Oct;27(10):4252-4263. doi: 10.1038/s41380-022-01656-x. Epub 2022 Jun 22. Mol Psychiatry. 2022. PMID: 35732693 Free PMC article.
-
Cellular and molecular mechanisms of aspartoacylase and its role in Canavan disease.Cell Biosci. 2024 Apr 6;14(1):45. doi: 10.1186/s13578-024-01224-6. Cell Biosci. 2024. PMID: 38582917 Free PMC article. Review.
-
Neuromuscular NMDA Receptors Modulate Developmental Synapse Elimination.J Neurosci. 2016 Aug 24;36(34):8783-9. doi: 10.1523/JNEUROSCI.1181-16.2016. J Neurosci. 2016. PMID: 27559162 Free PMC article.
-
Oxysterols in Central and Peripheral Synaptic Communication.Adv Exp Med Biol. 2024;1440:91-123. doi: 10.1007/978-3-031-43883-7_6. Adv Exp Med Biol. 2024. PMID: 38036877
-
To Break or to Brake Neuronal Network Accelerated by Ammonium Ions?PLoS One. 2015 Jul 28;10(7):e0134145. doi: 10.1371/journal.pone.0134145. eCollection 2015. PLoS One. 2015. PMID: 26217943 Free PMC article.
References
-
- Arun P, Madhavarao CN, Moffett JR, Namboodiri MAA. Regulation of N-acetylaspartate and N-acetylaspartylglutamate biosynthesis by protein kinase activators. J Neurochem. 2006;98:2034–2042. - PubMed
-
- Berger UV, Carter RE, Coyle JT. The immunocytochemical localization of N-acetylaspartyl glutamate, its hydrolysing enzyme NAALADase, and the NMDAR-1 receptor at a vertebrate neuromuscular junction. Neuroscience. 1995;64:847–850. - PubMed
-
- Berger UV, Carter RE, McKee M, Coyle JT. N-acetylated alpha-linked acidic dipeptidase is expressed by non-myelinating Schwann cells in the peripheral nervous system. J Neurocytol. 1995;24:99–109. - PubMed
-
- Berger UV, Luthi-Carter R, Passani LA, Elkabes S, Black I, Konradi C, Coyle JT. Glutamate carboxypeptidase II is expressed by astrocytes in the adult rat nervous system. J Comp Neurol. 1999;415:52–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

