Characterisation of dissolved organic compounds in hydrothermal fluids by stir bar sorptive extraction - gas chomatography - mass spectrometry. Case study: the Rainbow field (36°N, Mid-Atlantic Ridge)
- PMID: 23134621
- PMCID: PMC3518226
- DOI: 10.1186/1467-4866-13-8
Characterisation of dissolved organic compounds in hydrothermal fluids by stir bar sorptive extraction - gas chomatography - mass spectrometry. Case study: the Rainbow field (36°N, Mid-Atlantic Ridge)
Abstract
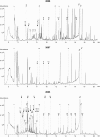
The analysis of the dissolved organic fraction of hydrothermal fluids has been considered a real challenge due to sampling difficulties, complexity of the matrix, numerous interferences and the assumed ppb concentration levels. The present study shows, in a qualitative approach, that Stir Bar Sorptive Extraction (SBSE) followed by Thermal Desorption - Gas Chromatography - Mass Spectrometry (TD-GC-MS) is suitable for extraction of small sample volumes and detection of a wide range of volatile and semivolatile organic compounds dissolved in hydrothermal fluids. In a case study, the technique was successfully applied to fluids from the Rainbow ultramafic-hosted hydrothermal field located at 36°14'N on the Mid-Atlantic Ridge (MAR). We show that n-alkanes, mono- and poly- aromatic hydrocarbons as well as fatty acids can be easily identified and their retention times determined. Our results demonstrate the excellent repeatability of the method as well as the possibility of storing stir bars for at least three years without significant changes in the composition of the recovered organic matter. A preliminary comparative investigation of the organic composition of the Rainbow fluids showed the great potential of the method to be used for assessing intrafield variations and carrying out time series studies. All together our results demonstrate that SBSE-TD-GC-MS analyses of hydrothermal fluids will make important contributions to the understanding of geochemical processes, geomicrobiological interactions and formation of mineral deposits.
Figures








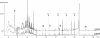

Similar articles
-
Multi-stir bar sorptive extraction for analysis of odor compounds in aqueous samples.J Chromatogr A. 2013 Nov 8;1315:70-9. doi: 10.1016/j.chroma.2013.09.070. Epub 2013 Sep 24. J Chromatogr A. 2013. PMID: 24094753
-
Stir bar sorptive extraction-thermal desorption-capillary GC-MS applied to biological fluids.Anal Bioanal Chem. 2002 May;373(1-2):46-55. doi: 10.1007/s00216-002-1260-8. Epub 2002 Mar 23. Anal Bioanal Chem. 2002. PMID: 12012171
-
Fractionated stir bar sorptive extraction using conventional and solvent-assisted approaches for enhanced identification capabilities of aroma compounds in beverages.J Chromatogr A. 2020 Sep 27;1628:461475. doi: 10.1016/j.chroma.2020.461475. Epub 2020 Aug 12. J Chromatogr A. 2020. PMID: 32822995
-
Analysis of 35 priority semivolatile compounds in water by stir bar sorptive extraction-thermal desorption-gas chromatography-mass spectrometry. I. Method optimisation.J Chromatogr A. 2003 May 30;999(1-2):91-101. doi: 10.1016/s0021-9673(03)00600-9. J Chromatogr A. 2003. PMID: 12885055
-
The production of methane, hydrogen, and organic compounds in ultramafic-hosted hydrothermal vents of the Mid-Atlantic Ridge.Astrobiology. 2015 May;15(5):381-99. doi: 10.1089/ast.2014.1198. Astrobiology. 2015. PMID: 25984920 Free PMC article. Review.
References
-
- Engel MH, Macko SA. Organic Geochemistry: Principles and Applications. Plenum Press, New York; 1993.
-
- Russell MJ, Hall AJ, Boyce AJ, Fallick AE. 100th Anniversary Special Paper: On Hydrothermal Convection Systems and the Emergence of Life. Econ Geol. 2005;100(3):419–438.
-
- Simoneit BRT, Lein AY, Peresypkin VI, Osipov GA. Composition and origin of hydrothermal petroleum and associated lipids in the sulfide deposits of the Rainbow field (Mid-Atlantic Ridge at 36°N). Geochim. Cosmochim. Acta. 2004;68(10):2275. doi: 10.1016/j.gca.2003.11.025. - DOI
-
- Delacour A, Früh-Green GL, Bernasconi SM, Schaeffer P, Kelley DS. Carbon geochemistry of serpentinites in the Lost City Hydrothermal System (30°N, MAR) Geochim Cosmochim Acta. 2008;72(15):3681–3702. doi: 10.1016/j.gca.2008.04.039. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

