Dioxin inhibits zebrafish epicardium and proepicardium development
- PMID: 23135548
- PMCID: PMC3551425
- DOI: 10.1093/toxsci/kfs301
Dioxin inhibits zebrafish epicardium and proepicardium development
Abstract
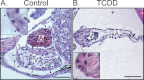
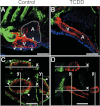
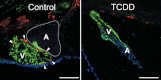
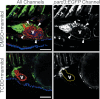
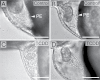
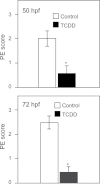
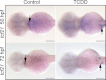
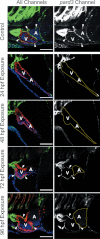
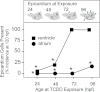
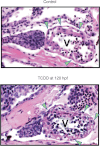
Embryonic exposure to the environmental contaminant and aryl hydrocarbon receptor agonist, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD, dioxin), disrupts cardiac development and function in fish, birds, and mammals. In zebrafish, the temporal window of sensitivity to the cardiotoxic effects of TCDD coincides with epicardium formation. We hypothesized that this TCDD-induced heart failure results from disruption of epicardial development. To determine whether embryonic TCDD exposure inhibits epicardium and proepicardium (PE) development in zebrafish, we used histology and fluorescence immunocytochemistry to examine the epicardium formation in fish exposed to TCDD. TCDD exposure prevented epicardium formation. Using live imaging and in situ hybridization, we found that TCDD exposure blocked the formation of the PE cluster. In situ hybridization experiments showed that TCDD exposure also prevented the expression of the PE marker tcf21 at the site where the PE normally forms. TCDD also inhibited expansion of the epicardial layer across the developing heart: Exposure after PE formation was completed prevented further expansion of the epicardium. However, TCDD exposure did not affect epicardial cells already present. Because TCDD blocks epicardium formation, but is not directly toxic to the epicardium once complete, we propose that inhibition of epicardium formation can account for the window of sensitivity to TCDD cardiotoxicity in developing zebrafish. Epicardium development is crucial to heart development. Loss of this layer during development may account for most if not all of the TCDD-induced cardiotoxicity in zebrafish.
Figures

References
-
- Antkiewicz D. S., Burns C. G., Carney S. A., Peterson R. E., Heideman W. (2005). Heart malformation is an early response to TCDD in embryonic zebrafish. Toxicol. Sci. 84, 368–377 - PubMed
-
- Belair C. D., Peterson R. E., Heideman W. (2001). Disruption of erythropoiesis by dioxin in the zebrafish. Dev. Dyn. 222, 581–594 - PubMed
-
- Brambilla G., Dellatte E., Fochi I., Iacovella N., Miniero R., di Domenico A. (2007). Depletion of selected polychlorinated biphenyl, dibenzodioxin, and dibenzofuran congeners in farmed rainbow trout (Oncorhynchus mykiss): A hint for safer fish farming. Chemosphere. 66, 1019–1030 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

