Gangliosides have a functional role during rotavirus cell entry
- PMID: 23135722
- PMCID: PMC3554060
- DOI: 10.1128/JVI.01964-12
Gangliosides have a functional role during rotavirus cell entry
Abstract
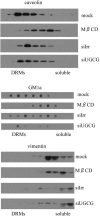
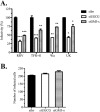
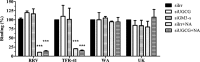
Cell entry of rotaviruses is a complex process, which involves sequential interactions with several cell surface molecules. Among the molecules implicated are gangliosides, glycosphingolipids with one or more sialic acid (SA) residues. The role of gangliosides in rotavirus cell entry was studied by silencing the expression of two key enzymes involved in their biosynthesis--the UDP-glucose:ceramide glucosyltransferase (UGCG), which transfers a glucose molecule to ceramide to produce glucosylceramide GlcCer, and the lactosyl ceramide-α-2,3-sialyl transferase 5 (GM3-s), which adds the first SA to lactoceramide-producing ganglioside GM3. Silencing the expression of both enzymes resulted in decreased ganglioside levels (as judged by GM1a detection). Four rotavirus strains tested (human Wa, simian RRV, porcine TFR-41, and bovine UK) showed a decreased infectivity in cells with impaired ganglioside synthesis; however, their replication after bypassing the entry step was not affected, confirming the importance of gangliosides for cell entry of the viruses. Interestingly, viral binding to the cell surface was not affected in cells with inhibited ganglioside synthesis, but the infectivity of all strains tested was inhibited by preincubation of gangliosides with virus prior to infection. These data suggest that rotaviruses can attach to cell surface in the absence of gangliosides but require them for productive cell entry, confirming their functional role during rotavirus cell entry.
Figures




Similar articles
-
Relative roles of GM1 ganglioside, N-acylneuraminic acids, and α2β1 integrin in mediating rotavirus infection.J Virol. 2014 Apr;88(8):4558-71. doi: 10.1128/JVI.03431-13. Epub 2014 Feb 5. J Virol. 2014. PMID: 24501414 Free PMC article.
-
Precursor of H-type II histo-blood group antigen and subterminal sialic acids on gangliosides are significantly implicated in cell entry and infection by a porcine P[11] rotavirus.Emerg Microbes Infect. 2025 Dec;14(1):2447608. doi: 10.1080/22221751.2024.2447608. Epub 2025 Jan 12. Emerg Microbes Infect. 2025. PMID: 39726161 Free PMC article.
-
Structure and function of a ganglioside receptor for porcine rotavirus.J Virol. 1998 Nov;72(11):9079-91. doi: 10.1128/JVI.72.11.9079-9091.1998. J Virol. 1998. PMID: 9765453 Free PMC article.
-
Molecular biology of rotavirus cell entry.Arch Med Res. 2002 Jul-Aug;33(4):356-61. doi: 10.1016/s0188-4409(02)00374-0. Arch Med Res. 2002. PMID: 12234525 Review.
-
Early events of rotavirus infection: the search for the receptor(s).Novartis Found Symp. 2001;238:47-60; discussion 60-3. doi: 10.1002/0470846534.ch4. Novartis Found Symp. 2001. PMID: 11444034 Review.
Cited by
-
Genome-wide RNAi screen reveals a role for the ESCRT complex in rotavirus cell entry.Proc Natl Acad Sci U S A. 2013 Jun 18;110(25):10270-5. doi: 10.1073/pnas.1304932110. Epub 2013 Jun 3. Proc Natl Acad Sci U S A. 2013. PMID: 23733942 Free PMC article.
-
Host Cell Response to Rotavirus Infection with Emphasis on Virus-Glycan Interactions, Cholesterol Metabolism, and Innate Immunity.Viruses. 2023 Jun 21;15(7):1406. doi: 10.3390/v15071406. Viruses. 2023. PMID: 37515094 Free PMC article.
-
Equine Rotavirus A under the One Health Lens: Potential Impacts on Public Health.Viruses. 2024 Jan 16;16(1):130. doi: 10.3390/v16010130. Viruses. 2024. PMID: 38257830 Free PMC article. Review.
-
Glycosphingolipid-Protein Interaction in Signal Transduction.Int J Mol Sci. 2016 Oct 15;17(10):1732. doi: 10.3390/ijms17101732. Int J Mol Sci. 2016. PMID: 27754465 Free PMC article. Review.
-
Ceramide and Related Molecules in Viral Infections.Int J Mol Sci. 2021 May 26;22(11):5676. doi: 10.3390/ijms22115676. Int J Mol Sci. 2021. PMID: 34073578 Free PMC article. Review.
References
-
- Lopez S, Arias CF. 2004. Multistep entry of rotavirus into cells: a Versaillesque dance. Trends Microbiol. 12:271–278 - PubMed
-
- Blanchard H, Yu X, Coulson BS, von Itzstein M. 2007. Insight into host cell carbohydrate-recognition by human and porcine rotavirus from crystal structures of the virion spike associated carbohydrate-binding domain (VP8*). J. Mol. Biol. 367:1215–1226 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

