Humoral immunity to smallpox vaccines and monkeypox virus challenge: proteomic assessment and clinical correlations
- PMID: 23135728
- PMCID: PMC3554095
- DOI: 10.1128/JVI.02089-12
Humoral immunity to smallpox vaccines and monkeypox virus challenge: proteomic assessment and clinical correlations
Abstract
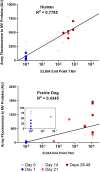
Despite the eradication of smallpox, orthopoxviruses (OPV) remain public health concerns. Efforts to develop new therapeutics and vaccines for smallpox continue through their evaluation in animal models despite limited understanding of the specific correlates of protective immunity. Recent monkeypox virus challenge studies have established the black-tailed prairie dog (Cynomys ludovicianus) as a model of human systemic OPV infections. In this study, we assess the induction of humoral immunity in humans and prairie dogs receiving Dryvax, Acam2000, or Imvamune vaccine and characterize the proteomic profile of immune recognition using enzyme-linked immunosorbent assays (ELISA), neutralization assays, and protein microarrays. We confirm anticipated similarities of antigenic protein targets of smallpox vaccine-induced responses in humans and prairie dogs and identify several differences. Subsequent monkeypox virus intranasal infection of vaccinated prairie dogs resulted in a significant boost in humoral immunity characterized by a shift in reactivity of increased intensity to a broader range of OPV proteins. This work provides evidence of similarities between the vaccine responses in prairie dogs and humans that enhance the value of the prairie dog model system as an OPV vaccination model and offers novel findings that form a framework for examining the humoral immune response induced by systemic orthopoxvirus infection.
Figures




References
-
- Breman JG, Arita I. 1980. The confirmation and maintenance of smallpox eradication. N. Engl. J. Med. 303:1263–1273 - PubMed
-
- Chapman JL, Nichols DK, Martinez MJ, Raymond JW. 2010. Animal models of orthopoxvirus infection. Vet. Pathol. 47:852–870 - PubMed
-
- Vorou RM, Papavassiliou VG, Pierroutsakos IN. 2008. Cowpox virus infection: an emerging health threat. Curr. Opin. Infect. Dis. 21:153–156 - PubMed
-
- Rimoin AW, Mulembakani PM, Johnston SC, Lloyd Smith JO, Kisalu NK, Kinkela TL, Blumberg S, Thomassen HA, Pike BL, Fair JN, Wolfe ND, Shongo RL, Graham BS, Formenty P, Okitolonda E, Hensley LE, Meyer H, Wright LL, Muyembe JJ. 2010. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc. Natl. Acad. Sci. U. S. A. 107:16262–16267 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

