Brief report: Mechanism of extravasation of infused stem cells
- PMID: 23135922
- PMCID: PMC3508398
- DOI: 10.1002/stem.1184
Brief report: Mechanism of extravasation of infused stem cells
Erratum in
- Stem Cells. 2013 Feb;31(2):415
Abstract
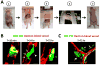
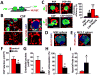
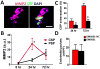
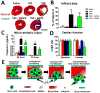
In order for bloodborne stem cells to be effective in tissue regeneration, cells must cross vessel walls and enter the parenchyma. Although such transmigration does occur, the mechanism remains elusive. Leukocytes invade tissue by diapedesis; stem cells are commonly assumed to do likewise, but evidence is lacking. Cardiac-derived regenerative cells and multicellular cardiospheres (CSPs) were infused into the coronary vessels of rat hearts. Serial histology revealed a novel mechanism of cell transmigration, "active vascular expulsion," which underlies the extravasation of infused cells and cell aggregates. In this mechanism, the vascular barrier undergoes extensive remodeling, while the cells themselves are relatively passive. The mechanism was confirmed in vivo by serial intravital microscopy of CSP extravasation in a dorsal skin flap model. Integrins and matrix metalloproteinases play critical roles in active vascular expulsion. In vitro models revealed that active vascular expulsion is generalizable to other stem cell types and to breast cancer cells. Recognition of active vascular expulsion as a mechanism for transvascular cell migration opens new opportunities to enhance the efficacy of vascularly delivered cell therapy.
Copyright © 2012 AlphaMed Press.
Conflict of interest statement
E.M. is a founder and equity holder in Capricor Inc. Capricor provided no funding for the present study. The remaining authors report no conflicts.
Figures


References
-
- Mooney DJ, Vandenburgh H. Cell delivery mechanisms for tissue repair. Cell Stem Cell. 2008;2:205–13. - PubMed
-
- Perin EC, Lopez J. Methods of stem cell delivery in cardiac diseases. Nat Clin Pract Cardiovasc Med. 2006;1:S110–113. - PubMed
-
- Steingen C, Brenig F, Baumgartner L, Schmidt J, Schmidt A, Bloch W. Characterization of key mechanisms in transmigration and invasion of mesenchymal stem cells. J Mol Cell Cardiol. 2008;44:1072–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

