Inhibition of T cell protein tyrosine phosphatase enhances interleukin-18-dependent hematopoietic stem cell expansion
- PMID: 23135963
- PMCID: PMC3593175
- DOI: 10.1002/stem.1276
Inhibition of T cell protein tyrosine phosphatase enhances interleukin-18-dependent hematopoietic stem cell expansion
Erratum in
- Stem Cells. 2013 Jun;31(6):1224. Tremblayef, Michel L [corrected to Tremblay, Michel L]
Abstract
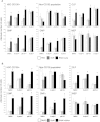
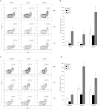
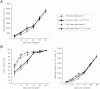
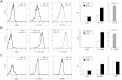
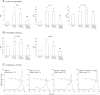
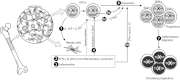
The clinical application of hematopoietic progenitor cell-based therapies for the treatment of hematological diseases is hindered by current protocols, which are cumbersome and have limited efficacy to augment the progenitor cell pool. We report that inhibition of T-cell protein tyrosine phosphatase (TC-PTP), an enzyme involved in the regulation of cytokine signaling, through gene knockout results in a ninefold increase in the number of hematopoietic progenitors in murine bone marrow (BM). This effect could be reproduced using a short (48 hours) treatment with a pharmacological inhibitor of TC-PTP in murine BM, as well as in human BM, peripheral blood, and cord blood. We also demonstrate that the ex vivo use of TC-PTP inhibitor only provides a temporary effect on stem cells and did not alter their capacity to reconstitute all hematopoietic components in vivo. We establish that one of the mechanisms whereby inhibition of TC-PTP mediates its effects involves the interleukin-18 (IL-18) signaling pathway, leading to increased production of IL-12 and interferon-gamma by progenitor cells. Together, our results reveal a previously unrecognized role for IL-18 in contributing to the augmentation of the stem cell pool and provide a novel and simple method to rapidly expand progenitor cells from a variety of sources using a pharmacological compound.
Copyright © 2012 AlphaMed Press.
Figures

References
-
- Mohle R, Kanz L. Hematopoietic growth factors for hematopoietic stem cell mobilization and expansion. Semin Hematol. 2007;44:193–202. - PubMed
-
- Ivanovic Z, Boiron JM. Ex vivo expansion of hematopoietic stem cells: Concept and clinical benefit. Transfus Clin Biol. 2009;16:489–500. - PubMed
-
- Trop S, Tremblay ML, Bourdeau A. Modulation of bone marrow-derived endothelial progenitor cell activity by protein tyrosine phosphatases. Trends Cardiovasc Med. 2008;18:180–186. - PubMed
-
- Bourdeau A, Dube N, Tremblay ML. Cytoplasmic protein tyrosine phosphatases, regulation and function: The roles of PTP1B and TC-PTP. Curr Opin Cell Biol. 2005;17:203–209. - PubMed
-
- Bourdeau A, Dube N, Heinonen KM, et al. TC-PTP-deficient bone marrow stromal cells fail to support normal B lymphopoiesis due to abnormal secretion of interferon-gamma. Blood. 2007;109:4220–4228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

