A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice
- PMID: 23136042
- PMCID: PMC8265389
- DOI: 10.1126/scitranslmed.3004249
A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice
Erratum in
-
Erratum for the Research Article: "A human disease model of drug toxicity-Induced pulmonary edema in alLung-on-a-chip microdevice" by D. Huh, D. C. Leslie, B. D. Matthews, J. P. Fraser, S. Jurek, G. A. Hamilton, K. S. Thorneloe, M. A. McAlexander, D. E. Ingber.Sci Transl Med. 2018 Jul 11;10(449):eaau4555. doi: 10.1126/scitranslmed.aau4555. Epub 2018 Jul 11. Sci Transl Med. 2018. PMID: 29997252 No abstract available.
Abstract
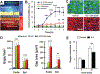
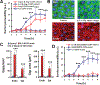
Preclinical drug development studies currently rely on costly and time-consuming animal testing because existing cell culture models fail to recapitulate complex, organ-level disease processes in humans. We provide the proof of principle for using a biomimetic microdevice that reconstitutes organ-level lung functions to create a human disease model-on-a-chip that mimics pulmonary edema. The microfluidic device, which reconstitutes the alveolar-capillary interface of the human lung, consists of channels lined by closely apposed layers of human pulmonary epithelial and endothelial cells that experience air and fluid flow, as well as cyclic mechanical strain to mimic normal breathing motions. This device was used to reproduce drug toxicity-induced pulmonary edema observed in human cancer patients treated with interleukin-2 (IL-2) at similar doses and over the same time frame. Studies using this on-chip disease model revealed that mechanical forces associated with physiological breathing motions play a crucial role in the development of increased vascular leakage that leads to pulmonary edema, and that circulating immune cells are not required for the development of this disease. These studies also led to identification of potential new therapeutics, including angiopoietin-1 (Ang-1) and a new transient receptor potential vanilloid 4 (TRPV4) ion channel inhibitor (GSK2193874), which might prevent this life-threatening toxicity of IL-2 in the future.
Conflict of interest statement
Figures

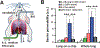
Comment in
-
Lung disorders: a new model and modulator of pulmonary oedema.Nat Rev Drug Discov. 2013 Jan;12(1):23. doi: 10.1038/nrd3917. Epub 2012 Dec 14. Nat Rev Drug Discov. 2013. PMID: 23237918 No abstract available.
-
Lung-on-a-chip microdevice, right ventricular dysfunction as a predictor of survival, and lung ultrasound in community-acquired pneumonia.Am J Respir Crit Care Med. 2013 Oct 15;188(8):1028-9. doi: 10.1164/rccm.201303-0469RR. Am J Respir Crit Care Med. 2013. PMID: 24127800 No abstract available.
References
-
- Pampaloni F, Reynaud EG, Stelzer EH, The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol 8, 839 (October, 2007). - PubMed
-
- Jang KJ, Suh KY, A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab Chip 10, 36 (January 7, 2010). - PubMed
-
- Lee PJ, Hung PJ, Lee LP, An artificial liver sinusoid with a microfluidic endothelial-like barrier for primary hepatocyte culture. Biotechnol Bioeng 97, 1340 (August 1, 2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

