Human melanoma metastasis in NSG mice correlates with clinical outcome in patients
- PMID: 23136044
- PMCID: PMC4501487
- DOI: 10.1126/scitranslmed.3004599
Human melanoma metastasis in NSG mice correlates with clinical outcome in patients
Abstract
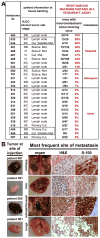
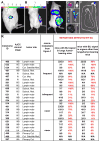
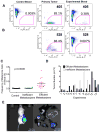
Studies of human cancer metastasis have been limited by a lack of experimental assays in which cancer cells from patients metastasize in vivo in a way that correlates with clinical outcome. This makes it impossible to study intrinsic differences in the metastatic properties of cancers from different patients. We recently developed an assay in which human melanomas readily engraft in nonobese diabetic/severe combined immunodeficient interleukin-2 receptor-γ chain null (NSG) mice. We show that melanomas from 25 patients exhibited reproducible differences in the rate of spontaneous metastasis after transplantation into NSG mice and that these differences correlated with clinical outcome in the patients. Stage IIIB/C melanomas that formed distant metastases within 22 months in patients also formed tumors that metastasized widely in NSG mice, whereas stage IIIB/C melanomas that did not form distant metastases within 22 to 50 months in patients metastasized more slowly in NSG mice. These differences in the efficiency of metastasis correlated with the presence of circulating melanoma cells in the blood of NSG mice, suggesting that the rate of entry into the blood is one factor that limits the rate of metastasis. The study of NSG mice can therefore yield information about the metastasis of human melanomas in vivo, in this case revealing intrinsic differences among stage III melanomas in their ability to circulate/survive in the blood and to metastasize.
Conflict of interest statement
Figures

References
-
- Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC, Jr, Morton DL, Ross MI, Sober AJ, Sondak VK. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009 Dec 20;27:6199–6206. - PMC - PubMed
-
- John T, Black MA, Toro TT, Leader D, Gedye CA, Davis ID, Guilford PJ, Cebon JS. Predicting clinical outcome through molecular profiling in stage III melanoma. Clin Cancer Res. 2008 Aug 15;14:5173–5180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

