Listeria monocytogenes-derived listeriolysin O has pathogen-associated molecular pattern-like properties independent of its hemolytic ability
- PMID: 23136118
- PMCID: PMC3535771
- DOI: 10.1128/CVI.00488-12
Listeria monocytogenes-derived listeriolysin O has pathogen-associated molecular pattern-like properties independent of its hemolytic ability
Abstract
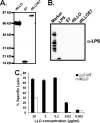
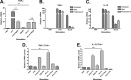
There is a constant need for improved adjuvants to augment the induction of immune responses against tumor-associated antigens (TAA) during immunotherapy. Previous studies have established that listeriolysin O (LLO), a cholesterol-dependent cytolysin derived from Listeria monocytogenes, exhibits multifaceted effects to boost the stimulation of immune responses to a variety of antigens. However, the direct ability of LLO as an adjuvant and whether it acts as a pathogen-associated molecular pattern (PAMP) have not been demonstrated. In this paper, we show that a detoxified, nonhemolytic form of LLO (dtLLO) is an effective adjuvant in tumor immunotherapy and may activate innate and cellular immune responses by acting as a PAMP. Our investigation of the adjuvant activity demonstrates that dtLLO, either fused to or administered as a mixture with a human papillomavirus type 16 (HPV-16) E7 recombinant protein, can augment antitumor immune responses and facilitate tumor eradication. Further mechanistic studies using bone marrow-derived dendritic cells suggest that dtLLO acts as a PAMP by stimulating production of proinflammatory cytokines and inducing maturation of antigen-presenting cells (APC). We propose that dtLLO is an effective adjuvant for tumor immunotherapy, and likely for other therapeutic settings.
Figures



Similar articles
-
Recombinant Listeria vaccines containing PEST sequences are potent immune adjuvants for the tumor-associated antigen human papillomavirus-16 E7.Cancer Res. 2004 Dec 15;64(24):8821-5. doi: 10.1158/0008-5472.CAN-04-1958. Cancer Res. 2004. PMID: 15604239
-
Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16.J Immunol. 2001 Dec 1;167(11):6471-9. doi: 10.4049/jimmunol.167.11.6471. J Immunol. 2001. PMID: 11714814
-
Evaluation of the safety and adjuvant effect of a detoxified listeriolysin O mutant on the humoral response to dengue virus antigens.Clin Exp Immunol. 2017 Apr;188(1):109-126. doi: 10.1111/cei.12906. Epub 2017 Jan 19. Clin Exp Immunol. 2017. PMID: 27886660 Free PMC article.
-
Listeriolysin O as a strong immunogenic molecule for the development of new anti-tumor vaccines.Hum Vaccin Immunother. 2013 May;9(5):1058-68. doi: 10.4161/hv.23871. Epub 2013 Feb 11. Hum Vaccin Immunother. 2013. PMID: 23399758 Free PMC article. Review.
-
Listeriolysin O: from bazooka to Swiss army knife.Philos Trans R Soc Lond B Biol Sci. 2017 Aug 5;372(1726):20160222. doi: 10.1098/rstb.2016.0222. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28630160 Free PMC article. Review.
Cited by
-
TRPV4-A Missing Link Between Mechanosensation and Immunity.Front Immunol. 2020 Mar 10;11:413. doi: 10.3389/fimmu.2020.00413. eCollection 2020. Front Immunol. 2020. PMID: 32210976 Free PMC article. Review.
-
Evolution of Listeria monocytogenes During a Persistent Human Prosthetic Hip Joint Infection.Front Microbiol. 2020 Jul 28;11:1726. doi: 10.3389/fmicb.2020.01726. eCollection 2020. Front Microbiol. 2020. PMID: 32849369 Free PMC article.
-
Clinical Experience and Recent Advances in the Development of Listeria-Based Tumor Immunotherapies.Front Immunol. 2021 Apr 14;12:642316. doi: 10.3389/fimmu.2021.642316. eCollection 2021. Front Immunol. 2021. PMID: 33936058 Free PMC article. Review.
-
More than a pore: the cellular response to cholesterol-dependent cytolysins.Toxins (Basel). 2013 Apr 12;5(4):618-36. doi: 10.3390/toxins5040618. Toxins (Basel). 2013. PMID: 23584137 Free PMC article. Review.
-
Multifaceted activity of listeriolysin O, the cholesterol-dependent cytolysin of Listeria monocytogenes.Subcell Biochem. 2014;80:161-95. doi: 10.1007/978-94-017-8881-6_9. Subcell Biochem. 2014. PMID: 24798012 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

