A recombinant immunotoxin against the tumor-associated antigen mesothelin reengineered for high activity, low off-target toxicity, and reduced antigenicity
- PMID: 23136186
- PMCID: PMC3546136
- DOI: 10.1158/1535-7163.MCT-12-0336
A recombinant immunotoxin against the tumor-associated antigen mesothelin reengineered for high activity, low off-target toxicity, and reduced antigenicity
Abstract
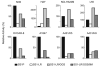
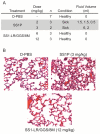
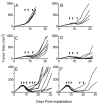
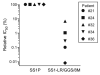
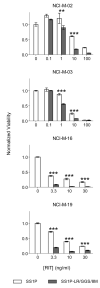
SS1P is a recombinant immunotoxin (RIT) engineered for the targeted elimination of malignant cells that express the tumor-associated antigen mesothelin. It is composed of an antimesothelin antibody variable fragment (Fv) linked to a cytotoxic fragment of Pseudomonas exotoxin A (PE) that includes domains II and III of native PE. The clinical use of SS1P is limited by its propensity to induce neutralizing antibodies and to cause a dose-limiting capillary leak syndrome (CLS) in patients. In this article, we describe a reengineered SS1P with improved properties that overcome these deficits. The redesign of SS1P consists of (i) removing the bulk of PE domain II (residues 251-273 and 284-394 of native PE), leaving only an 11-residue furin cleavage site, (ii) adding a Gly-Gly-Ser peptide linker after the furin cleavage site, and (iii) replacing eight highly solvent-exposed residues in the catalytic domain of PE. The new molecule, SS1-LR/GGS/8M, has cytotoxic activity comparable with SS1P on several mesothelin-expressing cell lines and remarkably improved activity on primary cells from patients with mesothelioma. In a mouse xenograft tumor model, high doses of SS1-LR/GGS/8M elicit antitumor activity superior to the activity of SS1P at its maximum-tolerated dose. In addition, SS1-LR/GGS/8M has greatly decreased ability to cause CLS in a rat model and reduced antigenicity or reactivity with antibodies to the sera of patients previously treated with SS1P.
Figures

References
-
- Kreitman RJ, Squires DR, Stetler-Stevenson M, Noel P, FitzGerald DJP, Wilson WH, et al. Phase I trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with B-cell malignancies. J Clin Oncol. 2005;23:6719–29. - PubMed
-
- Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kindler H, Willingham MC, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

