In vivo evidence for the crucial role of SF1 in steroid-producing cells of the testis, ovary and adrenal gland
- PMID: 23136395
- PMCID: PMC3509722
- DOI: 10.1242/dev.087247
In vivo evidence for the crucial role of SF1 in steroid-producing cells of the testis, ovary and adrenal gland
Abstract
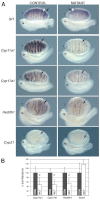
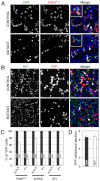
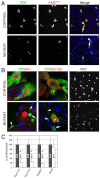
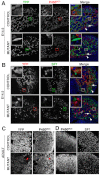
Adrenal and gonadal steroids are essential for life and reproduction. The orphan nuclear receptor SF1 (NR5A1) has been shown to regulate the expression of enzymes involved in steroid production in vitro. However, the in vivo role of this transcription factor in steroidogenesis has not been elucidated. In this study, we have generated steroidogenic-specific Cre-expressing mice to lineage mark and delete Sf1 in differentiated steroid-producing cells of the testis, the ovary and the adrenal gland. Our data show that SF1 is a regulator of the expression of steroidogenic genes in all three organs. In addition, Sf1 deletion leads to a radical change in cell morphology and loss of identity. Surprisingly, sexual development and reproduction in mutant animals were not compromised owing, in part, to the presence of a small proportion of SF1-positive cells. In contrast to the testis and ovary, the mutant adult adrenal gland showed a lack of Sf1-deleted cells and our studies suggest that steroidogenic adrenal cells during foetal stages require Sf1 to give rise to the adult adrenal population. This study is the first to show the in vivo requirements of SF1 in steroidogenesis and provides novel data on the cellular consequences of the loss of this protein specifically within steroid-producing cells.
Figures



References
-
- Buaas F. W., Val P., Swain A. (2009). The transcription co-factor CITED2 functions during sex determination and early gonad development. Hum. Mol. Genet. 18, 2989–3001 - PubMed
-
- Falender A. E., Lanz R., Malenfant D., Belanger L., Richards J. S. (2003). Differential expression of steroidogenic factor-1 and FTF/LRH-1 in the rodent ovary. Endocrinology 144, 3598–3610 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

