Neurons and cardiomyocytes derived from induced pluripotent stem cells as a model for mitochondrial defects in Friedreich's ataxia
- PMID: 23136396
- PMCID: PMC3634645
- DOI: 10.1242/dmm.010900
Neurons and cardiomyocytes derived from induced pluripotent stem cells as a model for mitochondrial defects in Friedreich's ataxia
Abstract
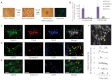
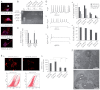
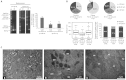
Friedreich's ataxia (FRDA) is a recessive neurodegenerative disorder commonly associated with hypertrophic cardiomyopathy. FRDA is due to expanded GAA repeats within the first intron of the gene encoding frataxin, a conserved mitochondrial protein involved in iron-sulphur cluster biosynthesis. This mutation leads to partial gene silencing and substantial reduction of the frataxin level. To overcome limitations of current cellular models of FRDA, we derived induced pluripotent stem cells (iPSCs) from two FRDA patients and successfully differentiated them into neurons and cardiomyocytes, two affected cell types in FRDA. All FRDA iPSC lines displayed expanded GAA alleles prone to high instability and decreased levels of frataxin, but no biochemical phenotype was observed. Interestingly, both FRDA iPSC-derived neurons and cardiomyocytes exhibited signs of impaired mitochondrial function, with decreased mitochondrial membrane potential and progressive mitochondrial degeneration, respectively. Our data show for the first time that FRDA iPSCs and their neuronal and cardiac derivatives represent promising models for the study of mitochondrial damage and GAA expansion instability in FRDA.
Figures



References
-
- Al-Mahdawi S., Pinto R. M., Varshney D., Lawrence L., Lowrie M. B., Hughes S., Webster Z., Blake J., Cooper J. M., King, R., et al. (2006). GAA repeat expansion mutation mouse models of Friedreich ataxia exhibit oxidative stress leading to progressive neuronal and cardiac pathology. Genomics 88, 580–590 - PMC - PubMed
-
- Calmels N., Schmucker S., Wattenhofer-Donzé M., Martelli A., Vaucamps N., Reutenauer L., Messaddeq N., Bouton C., Koenig M., Puccio H. (2009). The first cellular models based on frataxin missense mutations that reproduce spontaneously the defects associated with Friedreich ataxia. PLoS ONE 4, e6379. - PMC - PubMed
-
- Campuzano V., Montermini L., Moltò M. D., Pianese L., Cossée M., Cavalcanti F., Monros E., Rodius F., Duclos F., Monticelli A., et al. (1996). Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 271, 1423–1427 - PubMed
-
- Campuzano V., Montermini L., Lutz Y., Cova L., Hindelang C., Jiralerspong S., Trottier Y., Kish S. J., Faucheux B., Trouillas P., et al. (1997). Frataxin is reduced in Friedreich ataxia patients and is associated with mitochondrial membranes. Hum. Mol. Genet. 6, 1771–1780 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

