Stimulation of electro-olfactogram responses in the main olfactory epithelia by airflow depends on the type 3 adenylyl cyclase
- PMID: 23136416
- PMCID: PMC3506261
- DOI: 10.1523/JNEUROSCI.2180-12.2012
Stimulation of electro-olfactogram responses in the main olfactory epithelia by airflow depends on the type 3 adenylyl cyclase
Abstract
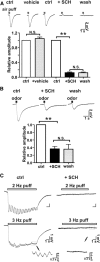
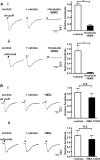
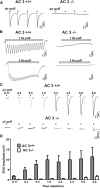
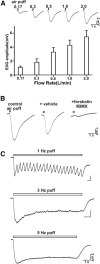
Cilia of olfactory sensory neurons are the primary sensory organelles for olfaction. The detection of odorants by the main olfactory epithelium (MOE) depends on coupling of odorant receptors to the type 3 adenylyl cyclase (AC3) in olfactory cilia. We monitored the effect of airflow on electro-olfactogram (EOG) responses and found that the MOE of mice can sense mechanical forces generated by airflow. The airflow-sensitive EOG response in the MOE was attenuated when cAMP was increased by odorants or by forskolin suggesting a common mechanism for airflow and odorant detection. In addition, the sensitivity to airflow was significantly impaired in the MOE from AC3(-/-) mice. We conclude that AC3 in the MOE is required for detecting the mechanical force of airflow, which in turn may regulate odorant perception during sniffing.
Figures




Similar articles
-
Acid-Sensing Ion Channels Contribute to Type III Adenylyl Cyclase-Independent Acid Sensing of Mouse Olfactory Sensory Neurons.Mol Neurobiol. 2020 Jul;57(7):3042-3056. doi: 10.1007/s12035-020-01943-0. Epub 2020 May 26. Mol Neurobiol. 2020. PMID: 32458389 Free PMC article.
-
Vomeronasal organ detects odorants in absence of signaling through main olfactory epithelium.Nat Neurosci. 2003 May;6(5):519-25. doi: 10.1038/nn1039. Nat Neurosci. 2003. PMID: 12665798
-
Maternal behavior is impaired in female mice lacking type 3 adenylyl cyclase.Neuropsychopharmacology. 2011 Mar;36(4):772-81. doi: 10.1038/npp.2010.211. Epub 2010 Dec 8. Neuropsychopharmacology. 2011. PMID: 21150908 Free PMC article.
-
Detection of odorants through the main olfactory epithelium and vomeronasal organ of mice.Nutr Rev. 2004 Nov;62(11 Pt 2):S189-92; discussion S224-41. doi: 10.1111/j.1753-4887.2004.tb00098.x. Nutr Rev. 2004. PMID: 15630934 Review.
-
Recording of the human electro-olfactogram.Physiol Behav. 2004 Oct 30;83(1):13-9. doi: 10.1016/j.physbeh.2004.07.024. Physiol Behav. 2004. PMID: 15501486 Review.
Cited by
-
Type 3 adenylyl cyclase: a key enzyme mediating the cAMP signaling in neuronal cilia.Int J Physiol Pathophysiol Pharmacol. 2016 Sep 30;8(3):95-108. eCollection 2016. Int J Physiol Pathophysiol Pharmacol. 2016. PMID: 27785336 Free PMC article. Review.
-
The cyclic AMP signaling pathway in the rodent main olfactory system.Cell Tissue Res. 2021 Jan;383(1):429-443. doi: 10.1007/s00441-020-03391-7. Epub 2021 Jan 15. Cell Tissue Res. 2021. PMID: 33447881 Review.
-
Ablation of Type III Adenylyl Cyclase in Mice Causes Reduced Neuronal Activity, Altered Sleep Pattern, and Depression-like Phenotypes.Biol Psychiatry. 2016 Dec 1;80(11):836-848. doi: 10.1016/j.biopsych.2015.12.012. Epub 2015 Dec 19. Biol Psychiatry. 2016. PMID: 26868444 Free PMC article.
-
International Union of Basic and Clinical Pharmacology. CI. Structures and Small Molecule Modulators of Mammalian Adenylyl Cyclases.Pharmacol Rev. 2017 Apr;69(2):93-139. doi: 10.1124/pr.116.013078. Pharmacol Rev. 2017. PMID: 28255005 Free PMC article. Review.
-
G protein-coupled odorant receptors underlie mechanosensitivity in mammalian olfactory sensory neurons.Proc Natl Acad Sci U S A. 2015 Jan 13;112(2):590-5. doi: 10.1073/pnas.1418515112. Epub 2014 Dec 30. Proc Natl Acad Sci U S A. 2015. PMID: 25550517 Free PMC article.
References
-
- Bakalyar HA, Reed RR. Identification of a specialized adenylyl cyclase that may mediate odorant detection. Science. 1990;250:1403–1406. - PubMed
-
- Boekhoff I, Inglese J, Schleicher S, Koch WJ, Lefkowitz RJ, Breer H. Olfactory desensitization requires membrane targeting of receptor kinase mediated by beta gamma-subunits of heterotrimeric G proteins. J Biol Chem. 1994;269:37–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
