Aversive stimuli differentially modulate real-time dopamine transmission dynamics within the nucleus accumbens core and shell
- PMID: 23136417
- PMCID: PMC3752139
- DOI: 10.1523/JNEUROSCI.3557-12.2012
Aversive stimuli differentially modulate real-time dopamine transmission dynamics within the nucleus accumbens core and shell
Abstract
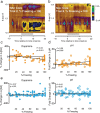
Although fear directs adaptive behavioral responses, how aversive cues recruit motivational neural circuitry is poorly understood. Specifically, while it is known that dopamine (DA) transmission within the nucleus accumbens (NAc) is imperative for mediating appetitive motivated behaviors, its role in aversive behavior is controversial. It has been proposed that divergent phasic DA transmission following aversive events may correspond to segregated mesolimbic dopamine pathways; however, this prediction has never been tested. Here, we used fast-scan cyclic voltammetry to examine real-time DA transmission within NAc core and shell projection systems in response to a fear-evoking cue. In male Sprague Dawley rats, we first demonstrate that a fear cue results in decreased DA transmission within the NAc core, but increased transmission within the NAc shell. We examined whether these changes in DA transmission could be attributed to modulation of phasic transmission evoked by cue presentation. We found that cue presentation decreased the probability of phasic DA release in the core, while the same cue enhanced the amplitude of release events in the NAc shell. We further characterized the relationship between freezing and both changes in DA as well as local pH. Although we found that both analytes were significantly correlated with freezing in the NAc across the session, changes in DA were not strictly associated with freezing while basic pH shifts in the core more consistently followed behavioral expression. Together, these results provide the first real-time neurochemical evidence that aversive cues differentially modulate distinct DA projection systems.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
