Somatosensory projections to cochlear nucleus are upregulated after unilateral deafness
- PMID: 23136418
- PMCID: PMC3501653
- DOI: 10.1523/JNEUROSCI.2598-12.2012
Somatosensory projections to cochlear nucleus are upregulated after unilateral deafness
Abstract
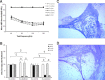
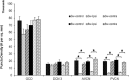
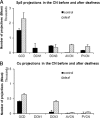
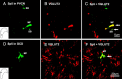
The cochlear nucleus (CN) receives innervation from auditory and somatosensory structures, which can be identified using vesicular glutamate transporters, VGLUT1 and VGLUT2. VGLUT1 is highly expressed in the magnocellular ventral CN (VCN), which receives auditory nerve inputs. VGLUT2 is predominantly expressed in the granule cell domain (GCD), which receives nonauditory inputs from somatosensory nuclei, including spinal trigeminal nucleus (Sp5) and cuneate nucleus (Cu). Two weeks after unilateral deafening VGLUT1 is significantly decreased in ipsilateral VCN while VGLUT2 is significantly increased in the ipsilateral GCD (Zeng et al., 2009), putatively reflecting decreased inputs from auditory nerve and increased inputs from nonauditory structures in guinea pigs. Here, we wished to determine whether the upregulation of VGLUT2 represents increases in the number of somatosensory projections to the CN that are maintained for longer periods of time. Thus, we examined concurrent changes in VGLUT levels and somatosensory projections in the CN using immunohistochemistry combined with anterograde tract tracing three and six weeks following unilateral deafening. The data reveal that unilateral deafness leads to increased numbers of VGLUT2-colabeled Sp5 and Cu projections to the ventral and dorsal CN. These findings suggest that Sp5 and Cu play significant and unique roles in cross-modal compensation and that, unlike after shorter term deafness, neurons in the magnocellular regions also participate in the compensation. The enhanced glutamatergic somatosensory projections to the CN may play a role in neural spontaneous hyperactivity associated with tinnitus.
Figures







Similar articles
-
Cuneate and spinal trigeminal nucleus projections to the cochlear nucleus are differentially associated with vesicular glutamate transporter-2.Neuroscience. 2011 Mar 10;176:142-51. doi: 10.1016/j.neuroscience.2010.12.010. Epub 2010 Dec 15. Neuroscience. 2011. PMID: 21167260 Free PMC article.
-
Cochlear damage changes the distribution of vesicular glutamate transporters associated with auditory and nonauditory inputs to the cochlear nucleus.J Neurosci. 2009 Apr 1;29(13):4210-7. doi: 10.1523/JNEUROSCI.0208-09.2009. J Neurosci. 2009. PMID: 19339615 Free PMC article.
-
Vessicular glutamate transporters 1 and 2 are differentially associated with auditory nerve and spinal trigeminal inputs to the cochlear nucleus.J Comp Neurol. 2007 Feb 1;500(4):777-87. doi: 10.1002/cne.21208. J Comp Neurol. 2007. PMID: 17154258
-
Plasticity of somatosensory inputs to the cochlear nucleus--implications for tinnitus.Hear Res. 2011 Nov;281(1-2):38-46. doi: 10.1016/j.heares.2011.05.001. Epub 2011 May 18. Hear Res. 2011. PMID: 21620940 Free PMC article. Review.
-
VGLUT1 and VGLUT2 mRNA expression in the primate auditory pathway.Hear Res. 2011 Apr;274(1-2):129-41. doi: 10.1016/j.heares.2010.11.001. Epub 2010 Nov 24. Hear Res. 2011. PMID: 21111036 Free PMC article. Review.
Cited by
-
Dorsal Cochlear Nucleus of the Rat: Representation of Complex Sounds in Ears Damaged by Acoustic Trauma.J Assoc Res Otolaryngol. 2015 Aug;16(4):487-505. doi: 10.1007/s10162-015-0522-z. Epub 2015 May 13. J Assoc Res Otolaryngol. 2015. PMID: 25967754 Free PMC article.
-
Maladaptive plasticity in tinnitus--triggers, mechanisms and treatment.Nat Rev Neurol. 2016 Mar;12(3):150-60. doi: 10.1038/nrneurol.2016.12. Epub 2016 Feb 12. Nat Rev Neurol. 2016. PMID: 26868680 Free PMC article. Review.
-
Underlying mechanisms of tinnitus: review and clinical implications.J Am Acad Audiol. 2014 Jan;25(1):5-22; quiz 126. doi: 10.3766/jaaa.25.1.2. J Am Acad Audiol. 2014. PMID: 24622858 Free PMC article. Review.
-
Reversing Cochlear Nucleus Maladaptive Plasticity via Customized Extracochlear Stimulation: A New Approach for Tinnitus Treatment.Adv Sci (Weinh). 2025 Mar;12(9):e2412349. doi: 10.1002/advs.202412349. Epub 2025 Jan 14. Adv Sci (Weinh). 2025. PMID: 39807573 Free PMC article.
-
Insult-induced adaptive plasticity of the auditory system.Front Neurosci. 2014 May 23;8:110. doi: 10.3389/fnins.2014.00110. eCollection 2014. Front Neurosci. 2014. PMID: 24904256 Free PMC article. Review.
References
-
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. - PubMed
-
- Bauer CA, Brozoski TJ, Myers K. Primary afferent dendrite degeneration as a cause of tinnitus. J Neurosci Res. 2007;85:1489–1498. - PubMed
-
- Benson CG, Gross JS, Suneja SK, Potashner SJ. Synaptophysin immunoreactivity in the cochlear nucleus after unilateral cochlear or ossicular removal. Synapse. 1997;25:243–257. - PubMed
-
- Brozoski TJ, Bauer CA. The effect of dorsal cochlear nucleus ablation on tinnitus in rats. Hear Res. 2005;206:227–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
