BMP4 sufficiency to induce choroid plexus epithelial fate from embryonic stem cell-derived neuroepithelial progenitors
- PMID: 23136431
- PMCID: PMC3505486
- DOI: 10.1523/JNEUROSCI.3227-12.2012
BMP4 sufficiency to induce choroid plexus epithelial fate from embryonic stem cell-derived neuroepithelial progenitors
Abstract
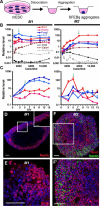
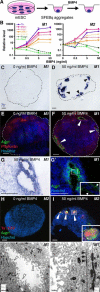
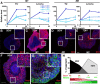
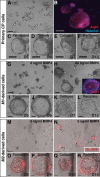
Choroid plexus epithelial cells (CPECs) have essential developmental and homeostatic roles related to the CSF and blood-CSF barrier they produce. Accordingly, CPEC dysfunction has been implicated in many neurological disorders, such as Alzheimer's disease, and transplant studies have provided proof-of-concept for CPEC-based therapies. However, such therapies have been hindered by the inability to expand or generate CPECs in culture. During development, CPECs differentiate from preneurogenic neuroepithelial cells and require bone morphogenetic protein (BMP) signaling, but whether BMPs suffice for CPEC induction is unknown. Here we provide evidence for BMP4 sufficiency to induce CPEC fate from neural progenitors derived from mouse embryonic stem cells (ESCs). CPEC specification by BMP4 was restricted to an early time period after neural induction in culture, with peak CPEC competency correlating to neuroepithelial cells rather than radial glia. In addition to molecular, cellular, and ultrastructural criteria, derived CPECs (dCPECs) had functions that were indistinguishable from primary CPECs, including self-assembly into secretory vesicles and integration into endogenous choroid plexus epithelium following intraventricular injection. We then used BMP4 to generate dCPECs from human ESC-derived neuroepithelial cells. These findings demonstrate BMP4 sufficiency to instruct CPEC fate, expand the repertoire of stem cell-derived neural derivatives in culture, and herald dCPEC-based therapeutic applications aimed at the unique interface between blood, CSF, and brain governed by CPECs.
Figures


References
-
- Caviness VS, Jr, Takahashi T, Nowakowski RS. Numbers, time and neocortical neuronogenesis: a general developmental and evolutionary model. Trends Neurosci. 1995;18:379–383. - PubMed
-
- Chauhan AN, Lewis PD. A quantitative study of cell proliferation in ependyma and choroid plexus in the postnatal rat brain. Neuropath Appl Neurobiol. 1979;5:303–309. - PubMed
-
- Chodobski A, Szmydynger-Chodobska J. Choroid plexus: target for polypeptides and site of their synthesis. Microsc Res Tech. 2001;52:65–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
