Membrane-proximal tryptophans of synaptobrevin II stabilize priming of secretory vesicles
- PMID: 23136435
- PMCID: PMC6621631
- DOI: 10.1523/JNEUROSCI.6282-11.2012
Membrane-proximal tryptophans of synaptobrevin II stabilize priming of secretory vesicles
Abstract
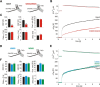
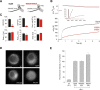
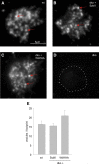
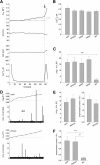
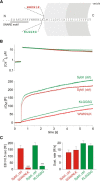
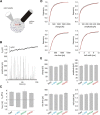
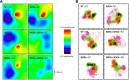
Trans-soluble N-ethylmaleimide-sensitive factor attachment protein (SNAP) receptor (SNARE) complexes formed between the SNARE motifs of synaptobrevin II, SNAP-25, and syntaxin play an essential role in Ca(2+)-regulated exocytosis. Apart from the well studied interactions of the SNARE domains, little is known about the functional relevance of other evolutionarily conserved structures in the SNARE proteins. Here, we show that substitution of two highly conserved tryptophan residues within the juxtamembrane domain (JMD) of the vesicular SNARE Synaptobrevin II (SybII) profoundly impairs priming of granules in mouse chromaffin cells without altering catecholamine release from single vesicles. Using molecular dynamic simulations of membrane-embedded SybII, we show that Trp residues of the JMD influence the electrostatic surface potential by controlling the position of neighboring lysine and arginine residues at the membrane-water interface. Our observations indicate a decisive role of the tryptophan moiety of SybII in keeping the vesicles in the release-ready state and support a model wherein tryptophan-mediated protein-lipid interactions assist in bridging the apposing membranes before fusion.
Figures




References
-
- Araç D, Chen X, Khant HA, Ubach J, Ludtke SJ, Kikkawa M, Johnson AE, Chiu W, Südhof TC, Rizo J. Close membrane-membrane proximity induced by Ca2+-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nat Struct Mol Biol. 2006;13:209–217. - PubMed
-
- Ashery U, Betz A, Xu T, Brose N, Rettig J. An efficient method for infection of adrenal chromaffin cells using the Semliki Forest virus gene expression system. Eur J Cell Biol. 1999;78:525–532. - PubMed
-
- Berendsen HJC, Postma JPM, Vangunsteren WF, Dinola A, Haak JR. Molecular dynamics with coupling to an external bath. J Chem Phys. 1984;81:3684–3690.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
