Hybrid capture II for high-risk human papillomavirus DNA testing to detect cervical precancerous lesions: A qualitative and quantitative study
- PMID: 23136614
- PMCID: PMC3490331
- DOI: 10.3892/etm_00000031
Hybrid capture II for high-risk human papillomavirus DNA testing to detect cervical precancerous lesions: A qualitative and quantitative study
Abstract
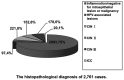
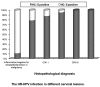
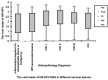
Hybrid capture II (HC-II) is the only technique that can be used in clinical human papillomavirus (HPV) DNA detection. However, there is controversy in regards to how to analyze and assess the viral load of high-risk (HR)-HPV by use of HC-II and the relation between viral load and cervical lesions. In this study, we analyzed the results of a sequential screening of outpatients at the Department of Obstetrics and Gynecology of the China-Japan Friendship Hospital, and we aimed to explore the relationship between HR-HPV viral load and the severity of cervical lesions, and to clarify the clinical significance of the titer of HR-HPV DNA determined by HC-II. Using HC-II, 2,761 women were screened for HR-HPV DNA combined with cytological testing using liquid-based cytology. All women with HR-HPV-positive results or abnormalities in cytology underwent a cervical biopsy through colposcopy. Cervical biopsies were taken in 1,051 women. The HR-HPV infection rate was 78.35% (76/97) in HPV-associated lesions, 87.33% (193/221) in cervical intraepithelial neoplasia (CIN) I, 94.74% (144/152) in CIN II, 100% (178/178) in CIN III and 100% (20/20) in invasive cervical cancer (ICC), respectively (P<0.05). Based on the criteria of histopathology, the sensitivity of HR-HPV DNA testing by HC-II for detecting high-grade cervical lesions was 97.71%, the specificity was 79.64%, the positive-predictive value was 41.06% and the negative-predictive value was 99.59%. The viral loads of HR-HPV DNA were 512.15±764.19 in HPV-associated lesions, 753.95±978.27 in CIN I, 871.08±1003.52 in CIN II, 603.40±740.25 in CIN III and 466.44±673.05 in ICC, respectively. In conclusion, the positive rate of HR-HPV increased significantly in accordance with the severity of cervical lesions. The viral loads of cervical inflammatory lesions were markedly lower than CINs and ICC. The viral loads of HR-HPV DNA tested by HC-II had no correlation with the grade of cervical lesions.
Figures
Similar articles
-
[Performance of vaginal self-sampling high-risk HPV genotyping as primary and combining cytology or viral load as secondary in cervical cancer screening].Zhonghua Fu Chan Ke Za Zhi. 2021 Apr 25;56(4):271-279. doi: 10.3760/cma.j.cn112141-20200824-00357. Zhonghua Fu Chan Ke Za Zhi. 2021. PMID: 33902239 Chinese.
-
Value of high‑risk human papillomavirus detection combined with colposcopy in the diagnosis of cervical cancer and precancerous lesions.Oncol Lett. 2024 Feb 29;27(4):185. doi: 10.3892/ol.2024.14318. eCollection 2024 Apr. Oncol Lett. 2024. PMID: 38476208 Free PMC article.
-
Evaluation of the detection of 14 high-risk human papillomaviruses with HPV 16 and HPV 18 genotyping for cervical cancer screening.Exp Ther Med. 2013 Nov;6(5):1332-1336. doi: 10.3892/etm.2013.1309. Epub 2013 Sep 18. Exp Ther Med. 2013. PMID: 24223668 Free PMC article.
-
High-risk human papillomavirus detection in women with low-grade squamous intraepithelial lesions or higher-grade cytology using the Cervista HPV HR test.J Low Genit Tract Dis. 2013 Jan;17(1):51-7. doi: 10.1097/LGT.0b013e31824ddbe0. J Low Genit Tract Dis. 2013. PMID: 22885641
-
Clinical applications and utility of ctDNA in cervical cancer and its precursor lesions: from screening to predictive biomarker.Cancer Cell Int. 2023 Dec 18;23(1):329. doi: 10.1186/s12935-023-03132-0. Cancer Cell Int. 2023. PMID: 38110977 Free PMC article. Review.
Cited by
-
Cytology versus HPV testing for cervical cancer screening in the general population.Cochrane Database Syst Rev. 2017 Aug 10;8(8):CD008587. doi: 10.1002/14651858.CD008587.pub2. Cochrane Database Syst Rev. 2017. PMID: 28796882 Free PMC article.
-
Human papillomavirus genotype prevalence in the women of Shanghai, China and its association with the severity of cervical neoplasia.Int J Clin Exp Pathol. 2018 Sep 1;11(9):4614-4621. eCollection 2018. Int J Clin Exp Pathol. 2018. PMID: 31949860 Free PMC article.
-
Evaluating the Performance of Hybrid Capture 2 Test as a Primary Screening Test from Studies Conducted in Low and Middle-Income Country Settings- Special Focus India.Asian Pac J Cancer Prev. 2021 Aug 1;22(8):2709-2716. doi: 10.31557/APJCP.2021.22.8.2709. Asian Pac J Cancer Prev. 2021. PMID: 34452578 Free PMC article.
References
-
- Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, Schiffman MH, Moreno V, Kurman R, Shah KV. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International Biological Study on Cervical Cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995;87:796–802. - PubMed
-
- Ying J, Lingya P. Role of high-risk human papillomavirus testing in the screening and management of cervical cancer precursors. Acta Acade Med Sinic. 2007;29:691–696. - PubMed
-
- Zhihong H, Deying Q, Ding W, Danhua H, Minjian C, Yanhong SH. Study of the relationship between loads of human papilloma virus in cervical carcinoma and cervical intraepithelial neoplasia. Matern Child Health Care Chin. 2006;21:1557–1559.
-
- Shumin L, Wenhua ZH, Lingying W, Fanghui ZH, Manni H, Nan L, Feng CH. Preliminary study on the relationship between loads of human papillomavirus in cervical carcinoma and cervical intraepithelial neoplasia. Chin J Obstet Gynecol. 2004;39:400–402. - PubMed
-
- Fanghui ZH, Junfei M, Youlin Q, Shoude R, Ling L, Wenhua ZH. Association between high-risk human papillomavirus DNA load and cervical intraepithelial lesion. Chin J Epidemiol. 2004;25:921–924. - PubMed
LinkOut - more resources
Full Text Sources