A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants
- PMID: 23136909
- PMCID: PMC10915853
- DOI: 10.1056/NEJMoa1208394
A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants
Abstract
Background: The candidate malaria vaccine RTS,S/AS01 reduced episodes of both clinical and severe malaria in children 5 to 17 months of age by approximately 50% in an ongoing phase 3 trial. We studied infants 6 to 12 weeks of age recruited for the same trial.
Methods: We administered RTS,S/AS01 or a comparator vaccine to 6537 infants who were 6 to 12 weeks of age at the time of the first vaccination in conjunction with Expanded Program on Immunization (EPI) vaccines in a three-dose monthly schedule. Vaccine efficacy against the first or only episode of clinical malaria during the 12 months after vaccination, a coprimary end point, was analyzed with the use of Cox regression. Vaccine efficacy against all malaria episodes, vaccine efficacy against severe malaria, safety, and immunogenicity were also assessed.
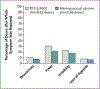
Results: The incidence of the first or only episode of clinical malaria in the intention-to-treat population during the 14 months after the first dose of vaccine was 0.31 per person-year in the RTS,S/AS01 group and 0.40 per person-year in the control group, for a vaccine efficacy of 30.1% (95% confidence interval [CI], 23.6 to 36.1). Vaccine efficacy in the per-protocol population was 31.3% (97.5% CI, 23.6 to 38.3). Vaccine efficacy against severe malaria was 26.0% (95% CI, -7.4 to 48.6) in the intention-to-treat population and 36.6% (95% CI, 4.6 to 57.7) in the per-protocol population. Serious adverse events occurred with a similar frequency in the two study groups. One month after administration of the third dose of RTS,S/AS01, 99.7% of children were positive for anti-circumsporozoite antibodies, with a geometric mean titer of 209 EU per milliliter (95% CI, 197 to 222).
Conclusions: The RTS,S/AS01 vaccine coadministered with EPI vaccines provided modest protection against both clinical and severe malaria in young infants. (Funded by GlaxoSmithKline Biologicals and the PATH Malaria Vaccine Initiative; RTS,S ClinicalTrials.gov number, NCT00866619.).
Figures

Comment in
-
Malaria vaccine trials--beyond efficacy end points.N Engl J Med. 2012 Dec 13;367(24):2349-51. doi: 10.1056/NEJMe1213392. Epub 2012 Nov 9. N Engl J Med. 2012. PMID: 23136910 No abstract available.
References
-
- World malaria report 2011. Geneva: World Health Organization, 2011.
-
- The RTS S Clinical Trials Partnership. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med 2011;365:1863–75. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
