5-Hydroxyindoles by intramolecular alkynol-furan diels-alder cycloaddition
- PMID: 23136970
- PMCID: PMC3684082
- DOI: 10.1021/jo3022605
5-Hydroxyindoles by intramolecular alkynol-furan diels-alder cycloaddition
Abstract
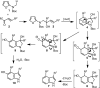
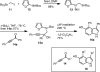
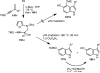
A convergent approach provides a convenient access to synthetically and biologically useful 3,4-disubstituted 5-hydroxyindoles. The one-pot procedure uses microwave heating to initiate an intramolecular [4 + 2]-cycloaddition of an alkynol segment onto a furan followed by a fragmentation, aromatization, and N-Boc deprotection cascade. Yields range from 15 to 74%, with aromatic substituents providing better conversions. 4-Trimethylsilylated analogues undergo a 1,3-silatropic rearrangement to give the O-TMS ethers.
Figures

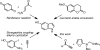

Similar articles
-
A microwave assisted intramolecular-furan-Diels-Alder approach to 4-substituted indoles.Chem Commun (Camb). 2009 Jan 7;(1):104-6. doi: 10.1039/b816989f. Epub 2008 Nov 11. Chem Commun (Camb). 2009. PMID: 19082013 Free PMC article.
-
An IMDAF cycloaddition approach toward the synthesis of the lycopodium alkaloid (±)-fawcettidine.J Org Chem. 2011 Apr 15;76(8):2753-61. doi: 10.1021/jo200125c. Epub 2011 Mar 10. J Org Chem. 2011. PMID: 21391623
-
Indole synthesis by palladium-catalyzed tandem allylic isomerization - furan Diels-Alder reaction.Org Biomol Chem. 2017 Aug 30;15(34):7093-7096. doi: 10.1039/c7ob01654a. Org Biomol Chem. 2017. PMID: 28813061
-
The Interplay between Kinetics and Thermodynamics in Furan Diels-Alder Chemistry for Sustainable Chemicals Production.Angew Chem Int Ed Engl. 2022 Apr 19;61(17):e202114720. doi: 10.1002/anie.202114720. Epub 2022 Feb 10. Angew Chem Int Ed Engl. 2022. PMID: 35014138 Free PMC article. Review.
-
Synthesis of Azulene Derivatives from 2H-Cyclohepta[b]furan-2-ones as Starting Materials: Their Reactivity and Properties.Int J Mol Sci. 2021 Oct 1;22(19):10686. doi: 10.3390/ijms221910686. Int J Mol Sci. 2021. PMID: 34639027 Free PMC article. Review.
Cited by
-
Eight-Step Enantioselective Total Synthesis of (-)-Cycloclavine.Angew Chem Int Ed Engl. 2017 Jan 2;56(1):324-327. doi: 10.1002/anie.201608820. Epub 2016 Nov 18. Angew Chem Int Ed Engl. 2017. PMID: 27860203 Free PMC article.
References
-
- Riendeau D, Aspiotis R, Ethier D, Gareau Y, Grimm EL, Guay J, Guiral S, Juteau H, Mancini JA, Méthot N, Rubin J, Friesen RW. Bioorg. Med. Chem. Lett. 2005;15:3352–3355. - PubMed
- Im G-YJ, Bronner SM, Goetz AE, Paton RS, Cheong PH-Y, Houk KN, Garg NK. J. Am. Chem. Soc. 2010;132:17933–17944. - PMC - PubMed
-
- Allen GR. Org. React. 1973;20:337–454.
- Humphrey GR, Kuethe JT. Chem. Rev. 2006;106:2875–2911. - PubMed
- Velezheva VS, Sokolov AI, Kornienko AG, Lyssenko KA, Nelyubina YV, Godovikov IA, Peregudov AS, Mironov AF. Tetrahedron Lett. 2008;49:7106–7109.
-
- Stoffman EJL, Clive DL. J. Org. Biomol. Chem. 2009;7:4862–4870. and references cited therein. - PubMed
-
- Ezquerra J, Pedregal C, Lamas C, Barluenga J, Pérez M, Garcia-Martin MA, González JM. J. Org. Chem. 1996;61:5804–5812.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

