Lipid modified triblock PAMAM-based nanocarriers for siRNA drug co-delivery
- PMID: 23137395
- PMCID: PMC3511617
- DOI: 10.1016/j.biomaterials.2012.10.024
Lipid modified triblock PAMAM-based nanocarriers for siRNA drug co-delivery
Abstract
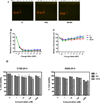
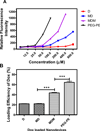
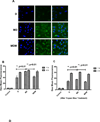
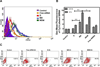
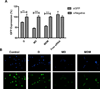
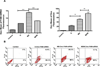
RNA interference by small interfering RNA (siRNA) holds promise to attenuate production of specific target proteins but is challenging in practice owing to the barriers for its efficient intracellular delivery. We have synthesized a triblock co-polymeric system, poly(amidoamine) dendrimer (generation 4)-poly(ethylene glycol)-1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (G(4)-D-PEG-(2K)-DOPE). G(4)-PAMAM dendrimer was utilized as a cationic source for efficient siRNA condensation; DOPE provided optimum hydrophobicity and compatible cellular interaction for enhanced cell penetration; PEG rendered flexibility to the G(4)-D for easy accessibility of siRNA for condensation; PEG-DOPE system provided stable micellization in a mixed micellar system. G(4)-D-PEG-(2K)-DOPE was incorporated into the self-assembled PEG-(5K)-PE micelles at a 1:1 molar ratio. Our results demonstrate that the modified dendrimer, G(4)-D-PEG-(2K)-DOPE and the micellar nanocarrier form stable polyplexes with siRNA, shows excellent serum stability and a significantly higher cellular uptake of siRNA that results in target protein down-regulation when compared to the G(4)-PAMAM dendrimer. Moreover, the mixed micellar system showed efficient micellization and higher drug (doxorubicin) loading efficiency. The G(4)-D-PEG-(2K)-DOPE has the higher efficacy for siRNA delivery, whereas G(4)-D-PEG-(2K)-DOPE/PEG-(5K)-PE micelles appear to be a promising carrier for drug/siRNA co-delivery, especially useful for the treatment of multi-drug resistant cancers.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures




Comment in
-
Nanocarriers for dual delivery of doxorubicin and siRNA to overcome multidrug resistance.Nanomedicine (Lond). 2013 Mar;8(3):329-30. doi: 10.2217/nnm.13.9. Nanomedicine (Lond). 2013. PMID: 23477333 No abstract available.
References
-
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411(6836):494–498. - PubMed
-
- Hannon GJ. RNA interference. Nature. 2002;418(6894):244–251. - PubMed
-
- Hannon GJ, Rossi JJ. Unlocking the potential of the human genome with RNA interference. Nature. 2004;431(7006):371–378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

