Management of trypanosomiasis and leishmaniasis
- PMID: 23137768
- PMCID: PMC3530408
- DOI: 10.1093/bmb/lds031
Management of trypanosomiasis and leishmaniasis
Abstract
Background: The current treatments for human African trypanosomiasis (HAT), Chagas disease and leishmaniasis (collectively referred to as the kinetoplastid diseases) are far from ideal but, for some, there has been significant recent progress. For HAT the only advances in treatment over the past two decades have been the introduction of an eflornithine/nifurtimox co-administration and a shorter regime of the old standard melarsoprol.
Sources of data: PubMed.
Areas of agreement: There is a need for new safe, oral drugs for cost-effective treatment of patients and use in control programmes for all the trypanosomatid diseases.
Areas of controversy: Cutaneous leishmaniasis is not on the agenda and treatments are lagging behind.
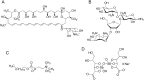
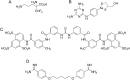
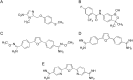
Growing points: There are three compounds in development for the treatment of the CNS stage of HAT: fexinidazole, currently due to entry into phase II clinical studies, a benzoxaborole (SCYX-7158) in phase I trials and a diamidine derivative (CPD-0802), in advanced pre-clinical development. For Chagas disease, two anti-fungal triazoles are now in clinical trial. In addition, clinical studies with benznidazole, a drug previously recommended only for acute stage treatment, are close to completion to determine the effectiveness in the treatment of early chronic and indeterminate Chagas disease. For visceral leishmaniasis new formulations, therapeutic switching, in particular AmBisome, and the potential for combinations of established drugs have significantly improved the opportunities for the treatment in the Indian subcontinent, but not in East Africa.
Areas timely for developing research: Improved diagnostic tools are needed to support treatment, for test of cure in clinical trials and for monitoring/surveillance of populations in control programmes.
Figures
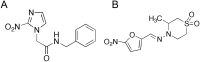
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

