Identification of small molecules that interfere with H1N1 influenza A viral replication
- PMID: 23139022
- PMCID: PMC3769975
- DOI: 10.1002/cmdc.201200453
Identification of small molecules that interfere with H1N1 influenza A viral replication
Abstract
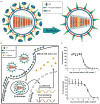
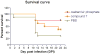
Successful replication of the influenza A virus requires both viral proteins and host cellular factors. In this study we used a cellular assay to screen for small molecules capable of interfering with any of such necessary viral or cellular components. We used an established reporter assay to assess influenza viral replication by monitoring the activity of co-expressed luciferase. We screened a diverse chemical compound library, resulting in the identification of compound 7, which inhibits a novel yet elusive target. Quantitative real-time PCR studies confirmed the dose-dependent inhibitory activity of compound 7 in a viral replication assay. Furthermore, we showed that compound 7 is effective in rescuing high-dose influenza infection in an in vivo mouse model. As oseltamivir-resistant influenza strains emerge, compound 7 could be further investigated as a new and potentially suitable scaffold for the development of anti-influenza agents that act on novel targets.
Copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
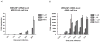
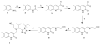
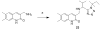
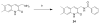
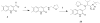
Similar articles
-
Antiviral activity of SA-2 against influenza A virus in vitro/vivo and its inhibition of RNA polymerase.Antiviral Res. 2016 Mar;127:68-78. doi: 10.1016/j.antiviral.2016.01.011. Epub 2016 Jan 21. Antiviral Res. 2016. PMID: 26802558
-
Screening of neurotransmitter receptor modulators reveals novel inhibitors of influenza virus replication.Front Cell Infect Microbiol. 2025 Apr 29;15:1562650. doi: 10.3389/fcimb.2025.1562650. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40365534 Free PMC article.
-
Total alkaloids from Alstonia scholaris inhibit influenza a virus replication and lung immunopathology by regulating the innate immune response.Phytomedicine. 2020 Oct;77:153272. doi: 10.1016/j.phymed.2020.153272. Epub 2020 Jun 29. Phytomedicine. 2020. PMID: 32702592
-
Metabolites of Seaweeds as Potential Agents for the Prevention and Therapy of Influenza Infection.Mar Drugs. 2019 Jun 22;17(6):373. doi: 10.3390/md17060373. Mar Drugs. 2019. PMID: 31234532 Free PMC article. Review.
-
Potential of small-molecule fungal metabolites in antiviral chemotherapy.Antivir Chem Chemother. 2017 Aug;25(2):20-52. doi: 10.1177/2040206617705500. Epub 2017 Jul 23. Antivir Chem Chemother. 2017. PMID: 28737040 Free PMC article. Review.
Cited by
-
High-Throughput cell-based immunofluorescence assays against influenza.SLAS Discov. 2024 Jan;29(1):66-76. doi: 10.1016/j.slasd.2023.10.008. Epub 2023 Nov 2. SLAS Discov. 2024. PMID: 37925159 Free PMC article.
-
Targeting Influenza A Virus RNA Promoter.Chem Biol Drug Des. 2015 Oct;86(4):663-73. doi: 10.1111/cbdd.12534. Epub 2015 Mar 13. Chem Biol Drug Des. 2015. PMID: 25676805 Free PMC article.
-
New-generation screening assays for the detection of anti-influenza compounds targeting viral and host functions.Antiviral Res. 2013 Oct;100(1):120-32. doi: 10.1016/j.antiviral.2013.07.018. Epub 2013 Aug 6. Antiviral Res. 2013. PMID: 23933115 Free PMC article. Review.
-
High-throughput screening for identification of influenza a inhibitors using a cell-based immunofluorescence assay.Antiviral Res. 2025 Aug;240:106209. doi: 10.1016/j.antiviral.2025.106209. Epub 2025 Jun 6. Antiviral Res. 2025. PMID: 40484321
-
Identification of Quinolinones as Antivirals against Venezuelan Equine Encephalitis Virus.Antimicrob Agents Chemother. 2021 Aug 17;65(9):e0024421. doi: 10.1128/AAC.00244-21. Epub 2021 Aug 17. Antimicrob Agents Chemother. 2021. PMID: 34152810 Free PMC article.
References
-
- Ciampor F, Bayley PM, Nermut MV, Hirst EM, Sugrue RJ, Hay AJ. Virology. 1992;188:14–24. - PubMed
-
- Konig R, Stertz S, Zhou Y, Inoue A, Hoffmann HH, Bhattacharyya S, Alamares JG, Tscherne DM, Ortigoza MB, Liang Y, Gao Q, Andrews SE, Bandyopadhyay S, De Jesus P, Tu BP, Pache L, Shih C, Orth A, Bonamy G, Miraglia L, Ideker T, Garcia-Sastre A, Young JA, Palese P, Shaw ML, Chanda SK. Nature. 2010;463:813–7. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

