Age-related susceptibility to apoptosis in human retinal pigment epithelial cells is triggered by disruption of p53-Mdm2 association
- PMID: 23139272
- PMCID: PMC4113331
- DOI: 10.1167/iovs.12-10495
Age-related susceptibility to apoptosis in human retinal pigment epithelial cells is triggered by disruption of p53-Mdm2 association
Abstract
Purpose: Relatively little is known about the contribution of p53/Mdm2 pathway in apoptosis of retinal pigment epithelial (RPE) cells or its possible link to dysfunction of aging RPE or to related blinding disorders such as age-related macular degeneration (AMD).
Methods: Age-associated changes in p53 activation were evaluated in primary RPE cultures from human donor eyes of various ages. Apoptosis was evaluated by activation of caspases and DNA fragmentation. Gene-specific small interfering RNA was used to knock down expression of p53.
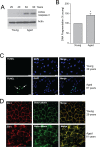
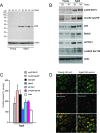
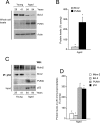
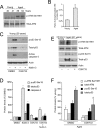
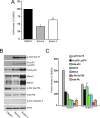
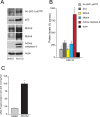
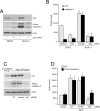
Results: We observed that the basal rate of p53-dependent apoptosis increased in an age-dependent manner in human RPE. The age-dependent increase in apoptosis was linked to alterations in several aspects of the p53 pathway. p53 phosphorylation Ser15 was increased through the stimulation of ATM-Ser1981. p53 acetylation Lys379 was increased through the inhibition of SIRT1/2. These two posttranslational modifications of p53 blocked the sequestration of p53 by Mdm2, thus resulting in an increase in free p53 and of p53 stimulation of apoptosis through increased expression of PUMA (p53 upregulated modulator of apoptosis) and activation of caspase-3. Aged RPE also had reduced expression of antiapoptotic Bcl-2, which contributed to the increase in apoptosis. Of particular interest in these studies was that pharmacologic treatments to block p53 phosphorylation, acetylation, or expression were able to protect RPE cells from apoptosis.
Conclusions: Our studies suggest that aging in the RPE leads to alterations of specific checkpoints in the apoptotic pathway, which may represent important molecular targets for the treatment of RPE-related aging disorders such as AMD.
Conflict of interest statement
Disclosure:
Figures


References
-
- Friedman DS, O'Colmain BJ, Muñoz B, et al. Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572 - PubMed
-
- Green WR. Histopathology of age-related macular degeneration. Mol Vis. 1999;3:5–27 - PubMed
-
- Nowak JZ. Age-related macular degeneration (AMD): pathogenesis and therapy. Pharmacol Rep. 2006;58:353–363 - PubMed
-
- Marmor MF, Wolfensberger TJ. The Retinal Pigment Epithelium: Function and Disease. New York: Oxford University Press; 1998
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

