Automated image analysis of skeletal muscle fiber cross-sectional area
- PMID: 23139362
- PMCID: PMC3544517
- DOI: 10.1152/japplphysiol.01022.2012
Automated image analysis of skeletal muscle fiber cross-sectional area
Abstract
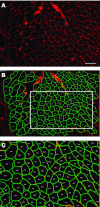
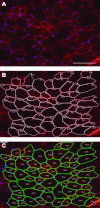
Morphological characteristics of muscle fibers, such as fiber size, are critical factors that determine the health and function of the muscle. However, at this time, quantification of muscle fiber cross-sectional area is still a manual or, at best, a semiautomated process. This process is labor intensive, time consuming, and prone to errors, leading to high interobserver variability. We have developed and validated an automatic image segmentation algorithm and compared it directly with commercially available semiautomatic software currently considered state of the art. The proposed automatic segmentation algorithm was evaluated against a semiautomatic method with manual annotation using 35 randomly selected cross-sectional muscle histochemical images. The proposed algorithm begins with ridge detection to enhance the muscle fiber boundaries, followed by robust seed detection based on concave area identification to find initial seeds for muscle fibers. The final muscle fiber boundaries are automatically delineated using a gradient vector flow deformable model. Our automatic approach is accurate and represents a significant advancement in efficiency; quantification of fiber area in muscle cross sections was reduced from 25-40 min/image to 15 s/image, while accommodating common quantification obstacles including morphological variation (e.g., heterogeneity in fiber size and fibrosis) and technical artifacts (e.g., processing defects and poor staining quality). Automatic quantification of muscle fiber cross-sectional area using the proposed method is a powerful tool that will increase sensitivity, objectivity, and efficiency in measuring muscle adaptation.
Figures







References
-
- Altman DG, Paton KA, Slavin G, Taylor AC, Ward P. Measurement of muscle fibre diameter by computer-aided image analysis. Int J Man-Machine Studies 14: 437–447, 1981
-
- Cooke R. Actomyosin interaction in striated muscle. Physiol Rev 77: 671–697, 1997 - PubMed
-
- Frangi AF, Niessen WJ, Vincken KL, Viergever MA, Colchester A, Wells WM, Delp S. Multiscale vessel enhancement filtering.. International Conference on Medical Image Computing and Computer-Assisted Intervention, Vol. 1496, p.130–37, 1998
-
- Koopman R, Zorenc AH, Gransier RJ, Cameron-Smith D, van Loon LJ. Increase in S6K1 phosphorylation in human skeletal muscle following resistance exercise occurs mainly in type II muscle fibers. Am J Physiol Endocrinol Metab 290: E1245–E1252, 2006 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

