Genetic analysis of the Hox hydrogenase in the cyanobacterium Synechocystis sp. PCC 6803 reveals subunit roles in association, assembly, maturation, and function
- PMID: 23139416
- PMCID: PMC3527937
- DOI: 10.1074/jbc.M112.392407
Genetic analysis of the Hox hydrogenase in the cyanobacterium Synechocystis sp. PCC 6803 reveals subunit roles in association, assembly, maturation, and function
Abstract
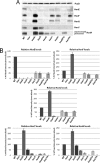
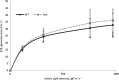
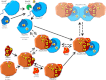
Hydrogenases are metalloenzymes that catalyze 2H(+) + 2e(-) ↔ H(2). A multisubunit, bidirectional [NiFe]-hydrogenase has been identified and characterized in a number of bacteria, including cyanobacteria, where it is hypothesized to function as an electron valve, balancing reductant in the cell. In cyanobacteria, this Hox hydrogenase consists of five proteins in two functional moieties: a hydrogenase moiety (HoxYH) with homology to heterodimeric [NiFe]-hydrogenases and a diaphorase moiety (HoxEFU) with homology to NuoEFG of respiratory Complex I, linking NAD(P)H ↔ NAD(P)(+) as a source/sink for electrons. Here, we present an extensive study of Hox hydrogenase in the cyanobacterium Synechocystis sp. PCC 6803. We identify the presence of HoxEFUYH, HoxFUYH, HoxEFU, HoxFU, and HoxYH subcomplexes as well as association of the immature, unprocessed large subunit (HoxH) with other Hox subunits and unidentified factors, providing a basis for understanding Hox maturation and assembly. The analysis of mutants containing individual and combined hox gene deletions in a common parental strain reveals apparent alterations in subunit abundance and highlights an essential role for HoxF and HoxU in complex/subcomplex association. In addition, analysis of individual and combined hox mutant phenotypes in a single strain background provides a clear view of the function of each subunit in hydrogenase activity and presents evidence that its physiological function is more complicated than previously reported, with no outward defects apparent in growth or photosynthesis under various growth conditions.
Figures





References
-
- Vignais P. M., Billoud B. (2007) Occurrence, classification, and biological function of hydrogenases. An overview. Chem. Rev. 107, 4206–4272 - PubMed
-
- Forzi L., Sawers R. G. (2007) Maturation of [NiFe]-hydrogenases in Escherichia coli. Biometals 20, 565–578 - PubMed
-
- Magalon A., Böck A. (2000) Dissection of the maturation reactions of the [NiFe] hydrogenase 3 from Escherichia coli taking place after nickel incorporation. FEBS Lett. 473, 254–258 - PubMed
-
- Carrieri D., Wawrousek K., Eckert C., Yu J., Maness P. C. (2011) The role of the bidirectional hydrogenase in cyanobacteria. Bioresour. Technol. 102, 8368–8377 - PubMed
-
- Appel J. (2012) The physiology and functional genomics of cyanobacterial hydrogenases and approaches towards biohydrogen production. in Functional Genomics and Evolution of Photosynthetic Systems (Burnap R., Vermaas W., eds) Illustrated Ed., pp. 357–381, Springer; Dordrecht, The Netherlands
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

