The phosphocholine-binding pocket on C-reactive protein is necessary for initial protection of mice against pneumococcal infection
- PMID: 23139417
- PMCID: PMC3522306
- DOI: 10.1074/jbc.M112.427310
The phosphocholine-binding pocket on C-reactive protein is necessary for initial protection of mice against pneumococcal infection
Abstract
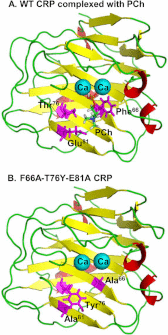
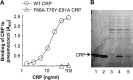
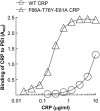
Human C-reactive protein (CRP) protects mice from lethal Streptococcus pneumoniae infection when injected into mice within the range of 6 h before to 2 h after the administration of pneumococci. Because CRP binds to phosphocholine-containing substances and subsequently activates the complement system, it has been proposed that the antipneumococcal function of CRP requires the binding of CRP to phosphocholine moieties present in pneumococcal cell wall C-polysaccharide. To test this proposal experimentally, in this study, we utilized a new CRP mutant incapable of binding to phosphocholine. Based on the structure of CRP-phosphocholine complexes, which showed that Phe(66), Thr(76), and Glu(81) formed the phosphocholine-binding pocket, we constructed a CRP mutant F66A/T76Y/E81A in which the pocket was blocked by substituting Tyr for Thr(76). When compared with wild-type CRP, mutant CRP bound more avidly to phosphoethanolamine and could be purified by affinity chromatography using phosphoethanolamine-conjugated Sepharose. Mutant CRP did not bind to phosphocholine, C-polysaccharide, or pneumococci. Mutant CRP was free in the mouse serum, and its rate of clearance in vivo was not faster than that of wild-type CRP. When either 25 μg or 150 μg of CRP was administered into mice, unlike wild-type CRP, mutant CRP did not protect mice from lethal pneumococcal infection. Mice injected with mutant CRP had higher mortality rates than mice that received wild-type CRP. Decreased survival was due to the increased bacteremia in mice treated with mutant CRP. We conclude that the phosphocholine-binding pocket on CRP is necessary for CRP-mediated initial protection of mice against lethal pneumococcal infection.
Figures





Similar articles
-
Protection against prolonged pneumococcal infection involves structural changes in C-reactive protein and subsequent binding to both phosphocholine and amyloids on the bacterial surface.Front Immunol. 2025 Jul 16;16:1631409. doi: 10.3389/fimmu.2025.1631409. eCollection 2025. Front Immunol. 2025. PMID: 40740789 Free PMC article.
-
C-reactive protein protects mice against pneumococcal infection via both phosphocholine-dependent and phosphocholine-independent mechanisms.Infect Immun. 2015 May;83(5):1845-52. doi: 10.1128/IAI.03058-14. Epub 2015 Feb 17. Infect Immun. 2015. PMID: 25690104 Free PMC article.
-
A C-reactive protein mutant that does not bind to phosphocholine and pneumococcal C-polysaccharide.J Immunol. 2002 Sep 15;169(6):3217-22. doi: 10.4049/jimmunol.169.6.3217. J Immunol. 2002. PMID: 12218140
-
Structure-Function Relationships of C-Reactive Protein in Bacterial Infection.Front Immunol. 2019 Feb 26;10:166. doi: 10.3389/fimmu.2019.00166. eCollection 2019. Front Immunol. 2019. PMID: 30863393 Free PMC article. Review.
-
The protective function of human C-reactive protein in mouse models of Streptococcus pneumoniae infection.Endocr Metab Immune Disord Drug Targets. 2008 Dec;8(4):231-7. doi: 10.2174/187153008786848321. Endocr Metab Immune Disord Drug Targets. 2008. PMID: 19075776 Free PMC article. Review.
Cited by
-
Evolution of C-Reactive Protein.Front Immunol. 2019 Apr 30;10:943. doi: 10.3389/fimmu.2019.00943. eCollection 2019. Front Immunol. 2019. PMID: 31114584 Free PMC article. Review.
-
Heterozygosity for transmembrane activator and calcium modulator ligand interactor A144E causes haploinsufficiency and pneumococcal susceptibility in mice.J Allergy Clin Immunol. 2017 Apr;139(4):1293-1301.e4. doi: 10.1016/j.jaci.2016.07.028. Epub 2016 Sep 5. J Allergy Clin Immunol. 2017. PMID: 27609654 Free PMC article.
-
C-Reactive Protein: Friend or Foe? Phylogeny From Heavy Metals to Modified Lipoproteins and SARS-CoV-2.Front Cardiovasc Med. 2022 Mar 24;9:797116. doi: 10.3389/fcvm.2022.797116. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35402541 Free PMC article. Review.
-
Treatment of Pneumococcal Infection by Using Engineered Human C-Reactive Protein in a Mouse Model.Front Immunol. 2020 Oct 7;11:586669. doi: 10.3389/fimmu.2020.586669. eCollection 2020. Front Immunol. 2020. PMID: 33117400 Free PMC article.
-
Pentraxins: structure, function, and role in inflammation.ISRN Inflamm. 2013 Sep 14;2013:379040. doi: 10.1155/2013/379040. ISRN Inflamm. 2013. PMID: 24167754 Free PMC article. Review.
References
-
- Kushner I., Samols D. (2011) Oswald Avery and the pneumococcus. Pharos Alpha Omega Alpha Honor Med. Soc. 74, 14–18 - PubMed
-
- Abernethy T. J., Avery O. T. (1941) The occurrence during acute infections of a protein not normally present in the blood. I. Distribution of the reactive protein in patients' sera and the effect of calcium on the flocculation reaction with C polysaccharide of pneumococcus. J. Exp. Med. 73, 173–182 - PMC - PubMed
-
- Volanakis J. E., Kaplan M. H. (1971) Specificity of C-reactive protein for choline phosphate residues of pneumococcal C-polysaccharide. Proc. Soc. Exp. Biol. Med. 136, 612–614 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

