Regulation of the SRC family kinases by Csk
- PMID: 23139636
- PMCID: PMC3492796
- DOI: 10.7150/ijbs.5141
Regulation of the SRC family kinases by Csk
Abstract
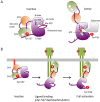
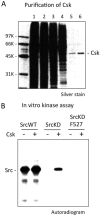
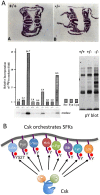
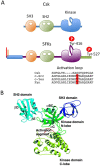
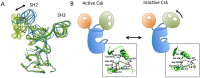
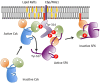
The non-receptor tyrosine kinase Csk serves as an indispensable negative regulator of the Src family tyrosine kinases (SFKs) by specifically phosphorylating the negative regulatory site of SFKs, thereby suppressing their oncogenic potential. Csk is primarily regulated through its SH2 domain, which is required for membrane translocation of Csk via binding to scaffold proteins such as Cbp/PAG1. The binding of scaffolds to the SH2 domain can also upregulate Csk kinase activity. These regulatory features have been elucidated by analyses of Csk structure at the atomic levels. Although Csk itself may not be mutated in human cancers, perturbation of the regulatory system consisting of Csk, Cbp/PAG1, or other scaffolds, and certain tyrosine phosphatases may explain the upregulation of SFKs frequently observed in human cancers. This review focuses on the molecular bases for the function, structure, and regulation of Csk as a unique regulatory tyrosine kinase for SFKs.
Keywords: Csk; Src family; tyrosine kinases.
Conflict of interest statement
Competing Interests: The author has declared that no competing interest exists.
Figures


References
-
- Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–65. - PubMed
-
- Brown M, Cooper J. Regulation, substrates and functions of src. Biochim Biophys Acta. 1996;1287:121–49. - PubMed
-
- Thomas S, Brugge J. Cellular Functions regulated by Src Family Kinases. Ann Rev Cell Biol. 1997;13:513–609. - PubMed
-
- Wilde A, Beattie EC, Lem L, Riethof DA, Liu SH, Mobley WC. et al. EGF receptor signaling stimulates SRC kinase phosphorylation of clathrin, influencing clathrin redistribution and EGF uptake. Cell. 1999;96:677–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

