Hyperglycemia-induced abnormalities in rat and human corneas: the potential of second harmonic generation microscopy
- PMID: 23139780
- PMCID: PMC3489670
- DOI: 10.1371/journal.pone.0048388
Hyperglycemia-induced abnormalities in rat and human corneas: the potential of second harmonic generation microscopy
Abstract
Background: Second Harmonic Generation (SHG) microscopy recently appeared as an efficient optical imaging technique to probe unstained collagen-rich tissues like cornea. Moreover, corneal remodeling occurs in many diseases and precise characterization requires overcoming the limitations of conventional techniques. In this work, we focus on diabetes, which affects hundreds of million people worldwide and most often leads to diabetic retinopathy, with no early diagnostic tool. This study then aims to establish the potential of SHG microscopy for in situ detection and characterization of hyperglycemia-induced abnormalities in the Descemet's membrane, in the posterior cornea.
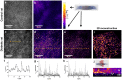
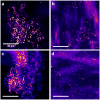
Methodology/principal findings: We studied corneas from age-matched control and Goto-Kakizaki rats, a spontaneous model of type 2 diabetes, and corneas from human donors with type 2 diabetes and without any diabetes. SHG imaging was compared to confocal microscopy, to histology characterization using conventional staining and transmitted light microscopy and to transmission electron microscopy. SHG imaging revealed collagen deposits in the Descemet's membrane of unstained corneas in a unique way compared to these gold standard techniques in ophthalmology. It provided background-free images of the three-dimensional interwoven distribution of the collagen deposits, with improved contrast compared to confocal microscopy. It also provided structural capability in intact corneas because of its high specificity to fibrillar collagen, with substantially larger field of view than transmission electron microscopy. Moreover, in vivo SHG imaging was demonstrated in Goto-Kakizaki rats.
Conclusions/significance: Our study shows unambiguously the high potential of SHG microscopy for three-dimensional characterization of structural abnormalities in unstained corneas. Furthermore, our demonstration of in vivo SHG imaging opens the way to long-term dynamical studies. This method should be easily generalized to other structural remodeling of the cornea and SHG microscopy should prove to be invaluable for in vivo corneal pathological studies.
Conflict of interest statement
Figures



Similar articles
-
Multimodal Highlighting of Structural Abnormalities in Diabetic Rat and Human Corneas.Transl Vis Sci Technol. 2013 Feb;2(2):3. doi: 10.1167/tvst.2.2.3. Epub 2013 Mar 4. Transl Vis Sci Technol. 2013. PMID: 24049714 Free PMC article.
-
[Structural analysis of normal corneas and diseased corneas by applying second harmonic generation].Nippon Ganka Gakkai Zasshi. 2011 Nov;115(11):1025-35. Nippon Ganka Gakkai Zasshi. 2011. PMID: 22171508 Japanese.
-
In vivo structural imaging of the cornea by polarization-resolved second harmonic microscopy.Biomed Opt Express. 2012 Jan 1;3(1):1-15. doi: 10.1364/BOE.3.000001. Epub 2011 Dec 1. Biomed Opt Express. 2012. PMID: 22254163 Free PMC article.
-
Protein conformation and molecular order probed by second-harmonic-generation microscopy.J Biomed Opt. 2012 Jun;17(6):060901. doi: 10.1117/1.JBO.17.6.060901. J Biomed Opt. 2012. PMID: 22734730 Review.
-
From nano to macro: studying the hierarchical structure of the corneal extracellular matrix.Exp Eye Res. 2015 Apr;133:81-99. doi: 10.1016/j.exer.2014.07.018. Exp Eye Res. 2015. PMID: 25819457 Free PMC article. Review.
Cited by
-
Corneal alteration and pathogenesis in diabetes mellitus.Int J Ophthalmol. 2019 Dec 18;12(12):1939-1950. doi: 10.18240/ijo.2019.12.17. eCollection 2019. Int J Ophthalmol. 2019. PMID: 31850180 Free PMC article. Review.
-
Chiral imaging of collagen by second-harmonic generation circular dichroism.Biomed Opt Express. 2013 May 20;4(6):909-16. doi: 10.1364/BOE.4.000909. Print 2013 Jun 1. Biomed Opt Express. 2013. PMID: 23761852 Free PMC article.
-
A systematic review on the impact of diabetes mellitus on the ocular surface.Nutr Diabetes. 2017 Mar 20;7(3):e251. doi: 10.1038/nutd.2017.4. Nutr Diabetes. 2017. PMID: 28319106 Free PMC article.
-
Risk factors for eye bank preparation failure of Descemet membrane endothelial keratoplasty tissue.Am J Ophthalmol. 2015 May;159(5):829-34.e2. doi: 10.1016/j.ajo.2015.01.030. Epub 2015 Jan 30. Am J Ophthalmol. 2015. PMID: 25640409 Free PMC article.
-
Fast monitoring of in-vivo conformational changes in myosin using single scan polarization-SHG microscopy.Biomed Opt Express. 2014 Nov 24;5(12):4362-73. doi: 10.1364/BOE.5.004362. eCollection 2014 Dec 1. Biomed Opt Express. 2014. PMID: 25574444 Free PMC article.
References
-
- Piston DW, Masters BR, Webb WW (1995) Three-dimensionally resolved nad(p)h cellular metabolic redox imaging of the in situ cornea with two-photon excitation laser scanning microscopy. J Microsc 178: 20–27. - PubMed
-
- Pena AM, Boulesteix T, Dartigalongue T, Schanne-Klein MC (2005) Chiroptical effects in the second harmonic signal of collagens i and iv. Journal of the American Chemical Society 127: 10314–10322. - PubMed
-
- Deniset-Besseau A, Duboisset J, Benichou E, Hache F, Brevet PF, et al. (2009) Measurement of the second-order hyperpolarizability of the collagen triple helix and determination of its physical origin. J Phys Chem B 113: 13437–13445. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

